Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection - PubMed (original) (raw)
Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection
Sumaira Z Hasnain et al. Gastroenterology. 2010 May.
Abstract
Background & aims: Hyperplasia of mucin-secreting intestinal goblet cells accompanies a number of enteric infections, including infections by nematode parasites. Nevertheless, the precise role of mucins in host defense in nematode infection is not known. We investigated the role of the mucin (Muc2) in worm expulsion and host immunity in a model of nematode infection.
Methods: Resistant (BALB/c, C57BL/6), susceptible (AKR), and Muc2-deficient mouse strains were infected with the nematode, Trichuris muris, and worm expulsion, energy status of the whipworms, changes in mucus/mucins, and inflammatory and immune responses were investigated after infection.
Results: The increase in Muc2 production, observed exclusively in resistant mice, correlated with worm expulsion. Moreover, expulsion of the worms from the intestine was significantly delayed in the Muc2-deficient mice. Although a marked impairment in the development of periodic acid Schiff (PAS)-stained intestinal goblet cells was observed in Muc2-deficient mice, as infection progressed a significant increase in the number of PAS-positive goblet cells was observed in these mice. Surprisingly, an increase in Muc5ac, a mucin normally expressed in the airways and stomach, was observed after infection of only the resistant animals. Overall, the mucus barrier in the resistant mice was less permeable than that of susceptible mice. Furthermore, the worms isolated from the resistant mice had a lower energy status.
Conclusions: Mucins are an important component of innate defense in enteric infection; this is the first demonstration of the important functional contribution of mucins to host protection from nematode infection.
Copyright 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
Supplementary Figure 1
PAS staining in the cecum showed a significant increase in goblet cell numbers only in the resistant (BALB/c) mice with infection (A). Worms are highlighted by arrows visible in the sections from susceptible mice. No main changes in goblet cell numbers in the colon of resistant (BALB/c) and susceptible (AKR) mice on day 14 and day 21 after infection compared with naïve (B). Representative of 3 mice. *P < .05.
Supplementary Figure 2
Expression of Tff3 (A) and Relm-β (B) were determined with the use of immunohistochemistry and RT-PCR in cecal tissue of Muc2 KO mice, and their resistant WT littermates on day 15 and day 20 after infection, respectively. RT-PCR showed no main changes in the mRNA expression of cell surface mucins, Muc1 (C), Muc4 (D), or Muc17 (E) in the WT and KO mice on day 20 after infection. Red dashed lines indicate naïve levels. Scale bar, 10 μm. Representative of 5 mice. *P < .05.
Supplementary Figure 3
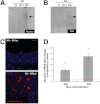
PAS staining with and without fast green counterstaining, and immunofluorescent staining with mMuc2 antibody of cecal tissue of Muc2 KO mice and their resistant WT littermates (A). Arrows highlight the emergence of smaller PAS-positive goblet cells in the Muc2 KO mice. Quantification of mMuc2 antibody staining represented as area stained in pixels per mm2 (B). RT-PCR confirms the increase in Muc2 levels after infection in resistant mice (C; red dashed line indicates naïve levels). Representative of 5 mice. *P < .05.
Supplementary Figure 4
(A) ATP production (data presented as relative light units per worm) was determined in the worms isolated from the resistant (BALB/c) or susceptible (AKR) mice compared with isolated worms transferred onto LS174T cell culture on day 19 after infection. (B) ATP production by worms isolated from Muc2-deficient mice and their WT littermates was determined on days 18 and 23 after infection. Representative of 3 mice. *P < .05.
Figure 1
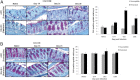
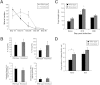
Worm burdens were assessed in both resistant (BALB/c) and susceptible (AKR) mice (A). Immunohistochemistry with mMuc2 antibody (B) and RT-PCR (C) were used to determine changes in Muc2 levels during infection. Nematodes are depicted by arrows (B). Red dashed line indicates naïve levels (C). Representative of 3 mice. Scale bar, 50 μm. *P < .05, **P < .01.
Figure 2
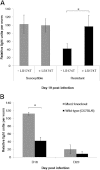
Muc2-deficient mice and their resistant WT (C57BL/6 background) littermates were infected orally with 300 eggs of T muris, and worm burdens were investigated on days 13, 15, 20, 25, and 30 after infection (A). Cytokine levels were determined in intestinal tissues (in pg/mg) or by concanavalin A stimulation of spleen cells (in pg/mL) (B). Cecal crypt length was measured (C), and crypt position of the highest BrdU+ cell (D) in Muc2-deficient and WT mice was determined. Representative of 5 mice. †P < .05 compared with day 13 after infection; *P < .05 compared with wild types. IFN-γ, interferon-γ.
Figure 3
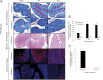
Quantification of goblet cell numbers in the cecum of WT and Muc2-deficient mice during infection (A); goblet cells marked by arrows in deficient mice can be visualized on day 30 after infection (PAS staining without fast green counterstain). Total mucus scraped from WT and Muc2-deficient mice were reduced/alkylated, separated by agarose gel electrophoresis, analyzed by Western blot, and probed with the mMuc2 antibody (B). The relative staining intensity of the mMuc2 antibody in the portion of the blot indicated by brackets was measured. A faint band (red box highlighted) was observed on day 21 after infection in the Muc2-deficient mice. The 2 Muc2 bands in the WT animals most likely represent the monomeric (●) and dimeric (▲) forms of Muc2 (B). Representative of 5 mice. *P < .05, **P < .01. ND indicates not detectable.
Figure 4
Muc5ac (A) and total glycoprotein (B) levels present in cecal mucus, determined by Western blotting using 45M1 antibody and PAS staining, respectively, in the Muc2-deficient mice. Immunofluorescence microscopy (C) and RT-PCR (D) illustrated Muc5ac was present in the Muc2-deficient mice after infection. D; Red dashed line = naïve levels. Representative of 5 mice. Scale bar; 10 μm. *P < .05, **P < .01.
Figure 5
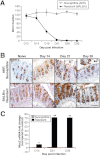
Muc5ac (A) and total glycoprotein (B) levels present in cecal mucus, determined by Western blotting with the use of 45M1 antibody and PAS staining, respectively, in the WT resistant (C57BL/6) mice. RT-PCR showed that Muc5ac levels increase significantly only in the resistant models (high-dose infection in BALB/c and C57BL/6 mice) and not in the susceptible models (low-dose infection in BALB/c and high-dose infection in AKR and SCID mice) (C; red dashed line indicates naïve levels). Immunofluorescence microscopy and immunohistochemistry showed that Muc5ac was present in some of the goblet cells of resistant mice after infection (D). Representative of 5 mice. Scale bar, 10 μm. *P < .05.
Figure 6
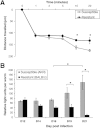
Fluorescent beads were used to determine the permeability of the mucus barrier of the susceptible (AKR) and resistant (BALB/c) mice on day 19 after infection, represented as the distance traveled from the top of the mucus barrier in the time stated (A). Energy levels (data presented as relative light units per worm) were determined in worms extracted from BALB/c and AKR mice during infection (B).
Similar articles
- New Role of Nod Proteins in Regulation of Intestinal Goblet Cell Response in the Context of Innate Host Defense in an Enteric Parasite Infection.
Wang H, Kim JJ, Denou E, Gallagher A, Thornton DJ, Shajib MS, Xia L, Schertzer JD, Grencis RK, Philpott DJ, Khan WI. Wang H, et al. Infect Immun. 2015 Nov 2;84(1):275-85. doi: 10.1128/IAI.01187-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26527214 Free PMC article. - Muc5ac: a critical component mediating the rejection of enteric nematodes.
Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK, Thornton DJ. Hasnain SZ, et al. J Exp Med. 2011 May 9;208(5):893-900. doi: 10.1084/jem.20102057. Epub 2011 Apr 18. J Exp Med. 2011. PMID: 21502330 Free PMC article. - Lactobacillus rhamnosus ingestion promotes innate host defense in an enteric parasitic infection.
McClemens J, Kim JJ, Wang H, Mao YK, Collins M, Kunze W, Bienenstock J, Forsythe P, Khan WI. McClemens J, et al. Clin Vaccine Immunol. 2013 Jun;20(6):818-26. doi: 10.1128/CVI.00047-13. Epub 2013 Mar 27. Clin Vaccine Immunol. 2013. PMID: 23536695 Free PMC article. - The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection.
Cliffe LJ, Grencis RK. Cliffe LJ, et al. Adv Parasitol. 2004;57:255-307. doi: 10.1016/S0065-308X(04)57004-5. Adv Parasitol. 2004. PMID: 15504540 Review. - Immune-mediated alteration in gut physiology and its role in host defence in nematode infection.
Khan WI, Collins SM. Khan WI, et al. Parasite Immunol. 2004 Aug-Sep;26(8-9):319-26. doi: 10.1111/j.0141-9838.2004.00715.x. Parasite Immunol. 2004. PMID: 15679628 Review.
Cited by
- Essential role of the electroneutral Na+-HCO3- cotransporter NBCn1 in murine duodenal acid-base balance and colonic mucus layer build-up in vivo.
Singh AK, Xia W, Riederer B, Juric M, Li J, Zheng W, Cinar A, Xiao F, Bachmann O, Song P, Praetorius J, Aalkjaer C, Seidler U. Singh AK, et al. J Physiol. 2013 Apr 15;591(8):2189-204. doi: 10.1113/jphysiol.2012.247874. Epub 2013 Feb 11. J Physiol. 2013. PMID: 23401617 Free PMC article. - Molecular and metabolomic changes in the proximal colon of pigs infected with Trichuris suis.
Dawson HD, Chen C, Li RW, Bell LN, Shea-Donohue T, Kringel H, Beshah E, Hill DE, Urban JF Jr. Dawson HD, et al. Sci Rep. 2020 Jul 30;10(1):12853. doi: 10.1038/s41598-020-69462-5. Sci Rep. 2020. PMID: 32732949 Free PMC article. - The Cysteine Protease Giardipain-1 from Giardia duodenalis Contributes to a Disruption of Intestinal Homeostasis.
Quezada-Lázaro R, Vázquez-Cobix Y, Fonseca-Liñán R, Nava P, Hernández-Cueto DD, Cedillo-Peláez C, López-Vidal Y, Huerta-Yepez S, Ortega-Pierres MG. Quezada-Lázaro R, et al. Int J Mol Sci. 2022 Nov 7;23(21):13649. doi: 10.3390/ijms232113649. Int J Mol Sci. 2022. PMID: 36362435 Free PMC article. - Role of mucins in lung homeostasis: regulated expression and biosynthesis in health and disease.
Symmes BA, Stefanski AL, Magin CM, Evans CM. Symmes BA, et al. Biochem Soc Trans. 2018 Jun 19;46(3):707-719. doi: 10.1042/BST20170455. Epub 2018 May 25. Biochem Soc Trans. 2018. PMID: 29802217 Free PMC article. Review. - Trichuris muris Model: Role in Understanding Intestinal Immune Response, Inflammation and Host Defense.
Yousefi Y, Haq S, Banskota S, Kwon YH, Khan WI. Yousefi Y, et al. Pathogens. 2021 Jul 22;10(8):925. doi: 10.3390/pathogens10080925. Pathogens. 2021. PMID: 34451389 Free PMC article. Review.
References
- Thornton D.J., Sheehan J.K. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. - PubMed
- Sheehan J.K., Thornton D.J. Heterogeneity and size distribution of gel-forming mucins. Methods Mol Biol. 2000;125:87–96. - PubMed
- Chang S.K., Dohrman A.F., Basbaum C.B. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology. 1994;107:28–36. - PubMed
- Tytgat K.M., Buller H.A., Opdam F.J., Kim Y.S., Einerhand A.W., Dekker J. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Gastroenterology. 1994;107:1352–1363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous