Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration - PubMed (original) (raw)
Review
Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration
David Finlay et al. Ann N Y Acad Sci. 2010 Jan.
Abstract
The established role for phosphatidylinositol (3,4,5) triphosphate (PI(3,4,5)P3) signaling pathways is to regulate cell metabolism. More recently it has emerged that PI(3,4,5)P3 signaling via mammalian target of rapamycin and Foxo transcription factors also controls lymphocyte trafficking by determining the repertoire of adhesion and chemokine receptors expressed by T lymphocytes. In quiescent T cells, nonphosphorylated active Foxos maintain expression of KLF2, a transcription factor that regulates expression of the chemokine receptors CCR7 and sphingosine 1 phosphate receptor, and the adhesion receptor CD62L that together control T-cell transmigration into secondary lymphoid tissues. PI(3,4,5)P3 mediates activation of protein kinase B, which phosphorylates and inactivates Foxos, thereby terminating expression of KLF2 and its target genes. The correct localization of lymphocytes is essential for effective immune responses, and the ability of phosphoinositide 3-kinase and mammalian target of rapamycin to regulate expression of chemokine receptors and adhesion molecules puts these signaling molecules at the core of the molecular mechanisms that control lymphocyte trafficking.
Figures
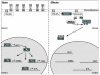
Figure 1. PI3-Kinase dependent activation of PKB and mTOR
Elevated PI(3,4,5)P3 following PI3-Kinase activation recruits PKB and its upstream activator, PDK1 to the membrane. Full activation of PKB requires the interation of its PH domain with PI(3,4,5)P3 followed by it phosphorylation on two key residues by PDK1 and another kinase, PDK2 which is cell type, context specific. PKB activates mTOR through dual mechanisms: (1) phosphorylation and inactivation of PRAS40, a negative regulator of mTOR and (2) inactivation of the TSC1/TSC2 complex which ihn turn promotes the inactivation of the positive regulator of mTOR, the small GTPase Rheb.
Figure 2. PKB/Foxo dependent regulation of KLF2 and its target genes
Naive T cells contain low levels of PI(3,4,5)P3 and active PKB. In these cells Foxo transcription factors are actively inducing the expression of targets genes such as KLF2. KLF2 in turn induces the expression of an array of genes that determine T cell trafficking, CD62L, CCR7 and S1P1. In contrast, in effector T cells, activated PI3-Kinase maintains elevated levels of PI(3,4,5)P3 and resultant activated PKB. PKB phosphorylates Foxo transcription factors on multiple residues maintaining them within the cytosol, thus repressing KLF2 expression and that of its target genes.
References
- Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. - PubMed
- Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. - PubMed
- Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. 2007;178:7632–7639. - PubMed
- Barbee SD, Alberola-Ila J. Phosphatidylinositol 3-kinase regulates thymic exit. J Immunol. 2005;174:1230–1238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous