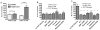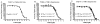Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice - PubMed (original) (raw)
Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice
Helen N Panas et al. J Neurosci Res. 2010 Jul.
Abstract
3-Iodothyronamine (T1AM) is a metabolite of thyroid hormone. It is an agonist at trace amine-associated receptor 1 (TAAR1), a recently identified receptor involved in monoaminergic regulation and a potential novel therapeutic target. Here, T1AM was studied using rhesus monkey TAAR1 and/or human dopamine transporter (DAT) co-transfected cells, and wild-type (WT) and TAAR1 knock-out (KO) mice. The IC(50) of T1AM competition for binding of the DAT-specific radio-ligand [(3)H]CFT was highly similar in DAT cells, WT striatal synaptosomes and KO striatal synaptosomes (0.72-0.81 microM). T1AM inhibition of 10 nM [(3)H]dopamine uptake (IC(50): WT, 1.4 + or - 0.5 microM; KO, 1.2 + or - 0.4 microM) or 50 nM [(3)H]serotonin uptake (IC(50): WT, 4.5 + or - 0.6 microM; KO, 4.7 + or - 1.1 microM) in WT and KO synaptosomes was also highly similar. Unlike other TAAR1 agonists that are DAT substrates, TAAR1 signaling in response to T1AM was not enhanced in the presence of DAT as determined by CRE-luciferase assay. In vivo, T1AM induced robust hypothermia in WT and KO mice equivalently and dose dependently (maximum change degrees Celsius: 50 mg/kg at 60 min: WT -6.0 + or - 0.4, KO -5.6 + or - 1.0; and 25 mg/kg at 30 min: WT -2.7 + or - 0.4, KO -3.0 + or - 0.2). Other TAAR1 agonists including beta-phenylethylamine (beta-PEA), MDMA (3,4-methylenedioxymethamphetamine) and methamphetamine also induced significant, time-dependent thermoregulatory responses that were alike in WT and KO mice. Therefore, TAAR1 co-expression does not alter T1AM binding to DAT in vitro nor T1AM inhibition of [(3)H]monoamine uptake ex vivo, and TAAR1 agonist-induced thermoregulatory responses are TAAR1-independent. Accordingly, TAAR1-directed compounds will likely not affect thermoregulation nor are they likely to be cryogens.
Figures
Figure 1. Activation of TAAR1 by T1AM and comparison to β-PEA
T1AM and β-PEA activation of TAAR1 and TAAR1-DAT cells was determined by a CRE-Luc reporter assay and are reported as percent change in relative light units (RLU). A. T1AM (10 μM) resulted in a 680% increase in RLU in TAAR1 cells versus a 760% RLU increase in TAAR1-DAT cells, whereas β-PEA (10 μM) resulted in a 531% increase in RLU in TAAR1 cells and 1750% increase in RLU response in TAAR1-DAT cells (n=3). B and C. TAAR1-DAT cells were exposed to various DAT blockers at (10 μM) or to vehicle (no DAT blocker), and were then treated with T1AM (10μM) or β-PEA (10μM). Pretreatment with DAT blockers had no significant effect on the response to 10 μM T1AM in TAAR1-DAT cells, whereas all DAT blockers significantly attenuated the enhanced response to 10 μM β-PEA in the TAAR1-DAT cells (n=3). All data shown are values of mean ± SEM. **: p < 0.01; ***: p < 0.001.
Figure 2. T1AM binding and uptake in cells and synaptosomes
A. T1AM binding to the dopamine transporter was measured by competitive binding versus [3H]CFT in DAT/HEK cells. The IC50 was 8.0±1.2 × 10-7 M (n=2). B. The IC50 of T1AM versus [3H]dopamine was 1.4±0.5 × 10-6 M for WT and 1.2±0.4 × 10-6 M for KO striatal synaptosomes (n=2). C. The IC50 of T1AM versus [3H]serotonin was 4.5±0.6 × 10-6 M for WT and 4.6±1.1 × 10-6 M for KO striatal synaptosomes (n=3). All data shown are values of mean ± SEM.
Figure 3. Thermoregulatory responses of T1AM, β-PEA, tyramine, MDMA, methamphetamine and saline in WT and KO mice
Wild type (WT) and TAAR1 knockout (KO) male mice were treated with 25 mg/kg or 50 mg/kg T1AM (A), 50 mg/kg β-PEA (B), 50 mg/kg tyramine (C), 25 mg/kg MDMA (D), 3 mg/kg methamphetamine (E) or saline (F). The number of mice in each group is indicated. Body temperature readings were captured using a wireless infra-red thermometer held over the shaved back area of each mouse at 5 minutes prior to the drug injection (-5) and at 15, 30, 45, 60, 75, 90, 120, 150, 180, 240, and 320 minutes post injection. Data was normalized to the saline controls (shown in F). A two-way ANOVA with repeated measures was used to determine if there was a significant difference in the thermoregulatory responses over all time points between WT and KO mice and across different drug treatments. There were no significant main or interactive effects. For each drug treatment, a one way ANOVA was used to determine statistical significance of individual time points versus the baseline (-5) temperature (detailed in supplemental Table S1). All data shown are values of mean ± SEM. *, p < 0.05 or lower versus the -5 time point for both WT and KO analyses except as indicated: in A, ns refers to KO data; in D and E, ns refers to WT data. See supplemental Table S1 for more detail.
Similar articles
- The Role of Biogenic Amine Transporters in Trace Amine-Associated Receptor 1 Regulation of Methamphetamine-Induced Neurotoxicity.
Miner NB, Phillips TJ, Janowsky A. Miner NB, et al. J Pharmacol Exp Ther. 2019 Oct;371(1):36-44. doi: 10.1124/jpet.119.258970. Epub 2019 Jul 18. J Pharmacol Exp Ther. 2019. PMID: 31320495 Free PMC article. - Beta-phenylethylamine alters monoamine transporter function via trace amine-associated receptor 1: implication for modulatory roles of trace amines in brain.
Xie Z, Miller GM. Xie Z, et al. J Pharmacol Exp Ther. 2008 May;325(2):617-28. doi: 10.1124/jpet.107.134247. Epub 2008 Jan 8. J Pharmacol Exp Ther. 2008. PMID: 18182557 - A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain.
Xie Z, Miller GM. Xie Z, et al. J Pharmacol Exp Ther. 2009 Jul;330(1):316-25. doi: 10.1124/jpet.109.153775. Epub 2009 Apr 13. J Pharmacol Exp Ther. 2009. PMID: 19364908 Free PMC article. - Trace amine-associated receptor 1: a multimodal therapeutic target for neuropsychiatric diseases.
Schwartz MD, Canales JJ, Zucchi R, Espinoza S, Sukhanov I, Gainetdinov RR. Schwartz MD, et al. Expert Opin Ther Targets. 2018 Jun;22(6):513-526. doi: 10.1080/14728222.2018.1480723. Epub 2018 Jun 5. Expert Opin Ther Targets. 2018. PMID: 29798691 Review. - The Case for TAAR1 as a Modulator of Central Nervous System Function.
Rutigliano G, Accorroni A, Zucchi R. Rutigliano G, et al. Front Pharmacol. 2018 Jan 10;8:987. doi: 10.3389/fphar.2017.00987. eCollection 2017. Front Pharmacol. 2018. PMID: 29375386 Free PMC article. Review.
Cited by
- TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity.
Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC. Revel FG, et al. Proc Natl Acad Sci U S A. 2011 May 17;108(20):8485-90. doi: 10.1073/pnas.1103029108. Epub 2011 Apr 27. Proc Natl Acad Sci U S A. 2011. PMID: 21525407 Free PMC article. - 3-Iodothyronamine, a Novel Endogenous Modulator of Transient Receptor Potential Melastatin 8?
Khajavi N, Mergler S, Biebermann H. Khajavi N, et al. Front Endocrinol (Lausanne). 2017 Aug 16;8:198. doi: 10.3389/fendo.2017.00198. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28861042 Free PMC article. Review. - Trace Amine-Associated Receptor 1 Regulation of Methamphetamine Intake and Related Traits.
Harkness JH, Shi X, Janowsky A, Phillips TJ. Harkness JH, et al. Neuropsychopharmacology. 2015 Aug;40(9):2175-84. doi: 10.1038/npp.2015.61. Epub 2015 Mar 5. Neuropsychopharmacology. 2015. PMID: 25740289 Free PMC article. - Avenues for the development of therapeutics that target trace amine associated receptor 1 (TAAR1).
Miller GM. Miller GM. J Med Chem. 2012 Mar 8;55(5):1809-14. doi: 10.1021/jm201437t. Epub 2012 Jan 20. J Med Chem. 2012. PMID: 22214431 Free PMC article. No abstract available. - Drug-induced hypothermia in stroke models: does it always protect?
Zhang M, Wang H, Zhao J, Chen C, Leak RK, Xu Y, Vosler P, Chen J, Gao Y, Zhang F. Zhang M, et al. CNS Neurol Disord Drug Targets. 2013 May 1;12(3):371-80. doi: 10.2174/1871527311312030010. CNS Neurol Disord Drug Targets. 2013. PMID: 23469851 Free PMC article. Review.
References
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966–71. - PMC - PubMed
- Branchek TA, Blackburn TP. Trace amine receptors as targets for novel therapeutics: legend, myth and fact. Curr Opin Pharmacol. 2003;3(1):90–7. - PubMed
- Braulke LJ, Klingenspor M, DeBarber A, Tobias SC, Grandy DK, Scanlan TS, Heldmaier G. 3-Iodothyronamine: a novel hormone controlling the balance between glucose and lipid utilisation. J Comp Physiol B. 2008;178(2):167–77. - PubMed
- Chiellini G, Frascarelli S, Ghelardoni S, Carnicelli V, Tobias SC, DeBarber A, Brogioni S, Ronca-Testoni S, Cerbai E, Grandy DK, Scanlan TS, Zucchi R. Cardiac effects of 3-iodothyronamine: a new aminergic system modulating cardiac function. FASEB J. 2007;21(7):1597–608. - PubMed
- Dhillo WS, Bewick GA, White NE, Gardiner JV, Thompson EL, Bataveljic A, Murphy KG, Roy D, Patel NA, Scutt JN, Armstrong A, Ghatei MA, Bloom SR. The thyroid hormone derivative 3-iodothyronamine increases food intake in rodents. Diabetes Obes Metab. 2009;11(3):251–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA025802/DA/NIDA NIH HHS/United States
- R03 DA025802/DA/NIDA NIH HHS/United States
- DK52798/DK/NIDDK NIH HHS/United States
- RR00168/RR/NCRR NIH HHS/United States
- R01 DA016606/DA/NIDA NIH HHS/United States
- R56 DK052798/DK/NIDDK NIH HHS/United States
- K02 DA025697/DA/NIDA NIH HHS/United States
- DA025697/DA/NIDA NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- DA016606/DA/NIDA NIH HHS/United States
- R01 DK052798/DK/NIDDK NIH HHS/United States
- K26 RR000168/RR/NCRR NIH HHS/United States
- DA022323/DA/NIDA NIH HHS/United States
- R21 DA022323/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials