Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates - PubMed (original) (raw)
Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates
Qing Xu et al. Neuron. 2010.
Abstract
Interneurons in the cerebral cortex regulate cortical functions through the actions of distinct subgroups that express parvalbumin, somatostatin, or calretinin. The genesis of the first two subgroups requires the expression of NKX2.1, which is maintained by SHH signaling during neurogenesis. In this paper, we report that mosaic elimination in the medial ganglionic eminence (MGE) of Smo, a key effector of SHH signaling, reveals that MGE progenitors retain a remarkable degree of plasticity during the neurogenic period. SHH signaling prevents the upregulation of GSX2 and conversion of some MGE progenitors to a caudal ganglionic eminence-like, bipolar calretinin-expressing cell fate that is promoted by GSX2. In addition, a higher level of SHH signaling promotes the generation of the somatostatin-expressing interneuron at the expense of parvalbumin-expressing subgroup. These results indicate that cortical interneuron diversity, a major determinant of cortical function, is critically influenced by differential levels of SHH signaling within the ventral telencephalon.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
Fig. 1. _Six3_Cre creates mosaic expression pattern in the MGE
(A) _Six3_Cre mediated GFP reporter expression in _Six3_Cre;ZEG mice is detectable in the medial ganglionic eminence (MGE) by E10.5. A few GFP+ cells are also observed occasionally in the lateral ganglionic eminence (LGE) and cortex (Ctx). Blue color is DAPI nuclear staining. (B) By E12.5 Cre reporter expression is present in radially oriented groups of cells in the MGE ventricular zone (VZ), as well as cells that appear to be migrating dorsally towards the striatum and cortex. (C) Higher magnification view of MGE from (B) shows GFP immunofluorescence signal. The arrow indicates the VZ region shown in (D) and (E). (D) Cre immunofluorescence and DAPI staining shows that, as implied by the Cre reporter expression, Cre protein is detected in many but not all MGE progenitors. (E) Confocal microscopy image of Cre protein and Cre reporter (GFP) expression in the MGE. Essentially all GFP+ cells also express detectable levels of Cre, although some Cre+ cells are not GFP+. (F) This panel shows the boxed area in (C). Some of the GFP+ cells appear to have down regulated Cre. However Cre+, GFP-negative cells are still present, consistent with previous results that reporter expression does not occur in all Cre+ cells in the ZEG mouse (Xu et al., 2008). Scale bar = 100 μm in (A) and (B); = 50 μm in (C); Scale bar = 20 μm in (D), (E) and (F).
Fig. 2. The MGE of _Six3_Cre mediated Smo conditional knockout embryos have reduced NKX2.1 and upregulated GSX2 expression
At E14.5 DAPI staining (A, A’) and labeling of S-phase cells by BrdU (B, B’) revealed no gross difference in brain size or proliferation in the _Six3_Cre;_Smo_f/f mutants. (C, C’) In contrast, the mutant embryo has a marked reduction of NKX2.1 immunofluorescence in subpopulations of MGE cells (arrow in C’, D’ and G’). (D, D’) Higher magnification view shows abnormal regions of BrdU+, NKX2.1-negative cells in the mutant MGE, suggesting that reduction of NKX2.1 in the ventricular zone is not due to premature cell cycle exit. (E-E’) GSX2 immunofluorescence shows upregulation in stripes of MGE progenitors of the _Six3_Cre;_Smo_f/f mutants (arrow in E’) compared to the _Six3_Cre;_Smo_f/+ heterozygous control. (F-H’). Colabeling of GSX2 (F, F’) and NKX2.1 (G, G’) reveals that regions in the mutant MGE with reduced NKX2.1 expression contain high expression of GSX2 (arrows in F’-H’; NKX2.1 and GSX2 merged image in H, H’). Scale bar = 200 μm in (A) and applies to (B-C’ and E, E’); Scale bar = 100 μm in (D) and applies to (D’); Scale bar = 10 μm in (F) and applies to (F’-H’).
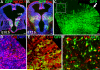
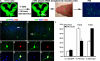
Fig. 3. The interneuron subgroups that express parvalbumin (PV) or somatostatin (SST) are differentially affected in the P25 cortex of _Six3_Cre;_Smo_f/f mice
In upper panel, coronal sections from the somatosensory (S1) cortex of controls (_Six3_Cre negative mice, A-C) are compared to mutants (A’-C’) for GABA expression (A, A’), PV (B, B’) and SST (C, C’). (D) Stereological quantification of cells per mm3 reveals significant reductions of GABA (in the deeper layers only) and PV (N=3, _t_-test). In contrast, the detection of SST+ and NPY+ (neuropeptide Y) interneuron subgroups is not affected. (E-F) To selectively examine the cell autonomous effect of loss of SHH signaling on interneuron fate determination in the MGE, triple immunolabeling for GFP, PV and SST was examined on P25 _Six3_Cre;_Smo_f/+;ZEG controls (E-E”), and _Six3_Cre;_Smo_f/f;ZEG mutants (F-F”) sections, and shown in cortical layers 5-6. Arrowheads indicate GFP+ cells that co-label with PV, and arrows indicate GFP+ cells that co-label with SST. (G) Areal densities of GFP+ interneurons co-labeled with PV or SST were significantly reduced in the mutant cortex (* indicates p<0.03, N=6; Wilcoxon signed rank tests). Scale bar = 100 μm in (A) and applies to (A’-C’). Scale bar = 50 μm in (E) and applies to (E’-F”).
Fig 4. Cell non-autonomous alteration of interneuron fate in the _Six3_Cre;_Smo_f/f mutants and ventral expansion of high SHH signaling
(A) Schema of experimental paradigm. The MGE is dissected from 250-μm thick vibratome sections of _Six3_Cre;_Smo_f/+;ZEG controls (B-D), or _Six3_Cre;_Smo_f/f;ZEG mutants (E-G). Cells are dissociated, and then plated on a feeder culture of neonatal cortex. Although some Cre+ progenitors fail to activate the GFP reporter (Fig. 1), in this paradigm the β-Gal+ population will primarily be composed of cells derived from Cre-negative (GFP-negative) progenitors (Novak et al., 2000). After 13 days the expression of SST (blue signal pseudocolored from Cy5 in C, D, F, G) by β-Gal+ cells (red) is assessed (arrowheads). (H) Counts of colabeling for β-Gal and SST reveals a two-fold increase of colabeled cells from the mutant MGE (_t_-test, N=3), suggesting that a cell non-autonomous effect of the _Six3_Cre;_Smo_f/f mutant results in overproduction of SST+ cells from the mutant MGE. (I, J, K) mRNA in situ hybridization for the SHH signaling targets Gli1, Ptch1, and Nkx6.2 in Cre-negative control E14.5 embryos are enriched in the dorsal-most region of the MGE. (I’, J’, K’) In sections from _Six3_Cre;_Smo_f/f mutants, these transcripts are expanded into ventral regions of the MGE (black arrows). Lack of co-labeling for Cre and either PTCH1 or Nkx6.2 (Supplemental Fig. 3) strongly suggests that this expansion is a cell non-autonomous effect. Scale bar = 25 μm in (B) and applies to (C-G). Scale bar = 200 μm in (I) and applies to (I’-K’).
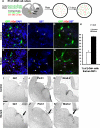
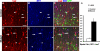
Fig 5. Exogenous SHH promotes ventral MGE progenitors into SST-expressing interneuron fates
(A) Telencephalic slices from E11.5 pan-GFP expressing embryos were cultured with or without 10nM of SHH ligand for 2 days. Retrovirus that encodes for nuclear localizing β-galactosidase (β-Gal) was added after 16 hours. The ventral (v) MGE cells were dissociated and transplanted into P1 neonatal cortex, and the brains were processed at P30 for immunohistochemistry. (B-K) Examples of co-labeling of GFP+ cells with PV, SST and β-Gal. Arrows in (B) and (C) indicate the triple-labeled cells that are magnified in the underneath panels, respectively. (L) The addition of SHH also did not significantly shift the percentage of transplanted cells co-labeling with β-Gal. However, the roughly 2:1 bias for vMGE progenitors to generate PV+ over SST+ interneurons in control transplants was dramatically reversed by exposure to exogenous SHH (P < 0.01, N=4, for both the PV decrease and SST increase with SHH treatment). Scale bar = 50 μm.
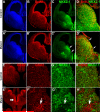
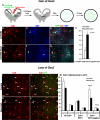
Fig. 6. _Smo_-recombined progenitors of the MGE can generate bipolar calretinin-expressing interneurons
Shown are images of superficial cortex from 40-μm coronal sections of P25 _Six3_Cre;_Smo_f/+;ZEG control (A-C) and _Six3_Cre;_Smo_f/f;ZEG mutant (D-F). (A-C) In the control section immunofluorescent colabeling of GFP and SST are indicated with short arrows, one multipolar cell weakly expressing Calr, GFP and SST is indicated with the long arrow. No vertically oriented Calr+ cells co-label with GFP, as this interneuron subgroup normally originates in the CGE, but not in the MGE (Butt et al., 2005; Xu et al., 2004). (D-F) The mutant section also contains GFP, SST and Calr triple-labeled cells with multipolar morphologies (long arrows), however, it contains several vertically oriented, GFP+ and Calr+ colabeled cells (arrowheads). The dramatic increase in the density of GFP+ cells that are vertically oriented, Calr+ and SST-negative in layers 2-3 of the mutant somatosensory cortex is quantified in (G) (t-test, P<0.01, N=5). To highlight cell morphology, the images were generated from Z-stacks collected using epifluorescence and a 10x objective, and then reconstructed using MetaMorph software. Scale bar = 50 μm in (A) and applies to (B-F).
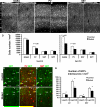
Fig. 7. GSX2 functions in the generation of bipolar calretinin-expressing interneurons
Panels (A-H) show results from a Gsx2 gain of function study. (A) E12.5 MGE progenitors were electroporated with pCAGIG (control, B-D) or pCAGIG-Gsx2 (E-G) plasmid. After one day, the MGE was dissociated and plated onto neonatal cortical cells and cultured for 13 days. GFP labels transfected cells. Image (B) and (C) are merged in (D), (E) and (F) are merged in (G). In controls, colabeling of Calr and SST is present in cells with multipolar morphology (arrow), but Calr labeling in bipolar neurons is rare. In contrast, transfection of pCAGIG-Gsx2 results in a more than two-fold increase in the percentage of GFP+ cells with a Calr+, bipolar phenotype (arrowhead in E and G; quantified in H; N=6). These cells do not express SST (F). (I-M) In the lower panel, the density of Calr+ interneurons in somatosensory cortex of conditional Gsx2 mutant mice (cKO; _Foxg1_-tTA;Tet-Cre;_Gsx2_f/f, K, L), are compared to the Cre-negative controls (I, J). Arrowheads indicate the vertically oriented Calr+, SST-negative interneurons. (M) Gsx2 cKO mice show a significant reduction of Calr+, SST-negative interneurons in both upper and lower layers (N=3). The relative density of PV+ or SST+ interneuron subgroups is not affected (not shown). Scale bar = 25 μm in (B-G), = 50 μm in (I) and applies to (J-L).
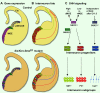
Fig. 8. Differential specification of cortical interneurons by SHH signaling and the homeodomain transcription factors NKX2.1 and GSX2
(A) Schema of NKX2.1 (purple), NKX6.2 (green), and GSX2 (blue) expression in the pallidal telencephalon at E13.5. In controls GSX2 is expressed at high levels in the dorsal region of the pallidal LGE and CGE, and weakly through the MGE. NKX6.2 is expressed along the MGE-LGE sulcus and dorsal-most MGE (dMGE), whereas NKX2.1 is expressed throughout the MGE. (B) Relationship of primary origin to interneuron fate. Most SST+ interneurons originate in the dorsal MGE, whereas most PV+ interneurons have a ventral MGE (vMGE) origin. Most bipolar, Calr+ interneurons originate from the CGE (not shown). In the _Six3_Cre;_Smo_f/f mutant, mosaic loss of SHH signaling results in cell autonomous loss of NKX2.1 expression in the MGE, and some of these cells upregulate GSX2. This conversion is associated with the abnormal production of bipolar, Calr+ interneurons from_Six3_-lineage MGE progenitors. At the same time, Cre-negative progenitors in the vMGE upregulate SHH signaling and Nkx6.2, converting their production to SST+ interneurons. (C) A two-state model for the role of SHH on interneuron fate determination in the MGE, after initial patterning has been established. High levels of SHH signaling in the dMGE drive NKX2.1+ progenitors to produce mainly SST+ interneurons, while lower levels of SHH signaling in the vMGE maintains the generation of the PV+ subgroup. On the other hand, bipolar Calr+ interneurons are generated by high GSX2+ progenitors in the CGE, that at this stage have become independent of SHH signaling (Machold et al., 2003).
Similar articles
- Duration of culture and sonic hedgehog signaling differentially specify PV versus SST cortical interneuron fates from embryonic stem cells.
Tyson JA, Goldberg EM, Maroof AM, Xu Q, Petros TJ, Anderson SA. Tyson JA, et al. Development. 2015 Apr 1;142(7):1267-78. doi: 10.1242/dev.111526. Development. 2015. PMID: 25804737 Free PMC article. - Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon.
Xu Q, Wonders CP, Anderson SA. Xu Q, et al. Development. 2005 Nov;132(22):4987-98. doi: 10.1242/dev.02090. Epub 2005 Oct 12. Development. 2005. PMID: 16221724 - Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence.
Inan M, Welagen J, Anderson SA. Inan M, et al. Cereb Cortex. 2012 Apr;22(4):820-7. doi: 10.1093/cercor/bhr148. Epub 2011 Jun 21. Cereb Cortex. 2012. PMID: 21693785 Free PMC article. - Cortical interneuron fate determination: diverse sources for distinct subtypes?
Xu Q, de la Cruz E, Anderson SA. Xu Q, et al. Cereb Cortex. 2003 Jun;13(6):670-6. doi: 10.1093/cercor/13.6.670. Cereb Cortex. 2003. PMID: 12764043 Review. - The development of MGE-derived cortical interneurons: An Lhx6 tale.
Christodoulou O, Maragkos I, Antonakou V, Denaxa M. Christodoulou O, et al. Int J Dev Biol. 2022;66(1-2-3):43-49. doi: 10.1387/ijdb.210185md. Int J Dev Biol. 2022. PMID: 34881792 Review.
Cited by
- Derivation and isolation of NKX2.1-positive basal forebrain progenitors from human embryonic stem cells.
Germain ND, Banda EC, Becker S, Naegele JR, Grabel LB. Germain ND, et al. Stem Cells Dev. 2013 May 15;22(10):1477-89. doi: 10.1089/scd.2012.0264. Epub 2013 Mar 5. Stem Cells Dev. 2013. PMID: 23351095 Free PMC article. - The Role of Inhibitory Interneurons in Circuit Assembly and Refinement Across Sensory Cortices.
Ferrer C, De Marco García NV. Ferrer C, et al. Front Neural Circuits. 2022 Apr 7;16:866999. doi: 10.3389/fncir.2022.866999. eCollection 2022. Front Neural Circuits. 2022. PMID: 35463203 Free PMC article. Review. - Annual Research Review: Development of the cerebral cortex: implications for neurodevelopmental disorders.
Rubenstein JL. Rubenstein JL. J Child Psychol Psychiatry. 2011 Apr;52(4):339-55. doi: 10.1111/j.1469-7610.2010.02307.x. Epub 2010 Aug 24. J Child Psychol Psychiatry. 2011. PMID: 20735793 Free PMC article. Review. - Sonic hedgehog functions through dynamic changes in temporal competence in the developing forebrain.
Sousa VH, Fishell G. Sousa VH, et al. Curr Opin Genet Dev. 2010 Aug;20(4):391-9. doi: 10.1016/j.gde.2010.04.008. Epub 2010 May 11. Curr Opin Genet Dev. 2010. PMID: 20466536 Free PMC article. Review. - Sonic hedgehog signaling regulates mode of cell division of early cerebral cortex progenitors and increases astrogliogenesis.
Araújo GL, Araújo JA, Schroeder T, Tort AB, Costa MR. Araújo GL, et al. Front Cell Neurosci. 2014 Mar 11;8:77. doi: 10.3389/fncel.2014.00077. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24653675 Free PMC article.
References
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. - PubMed
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. - PubMed
- Cavanagh ME, Parnavelas JG. Development of vasoactive-intestinal-polypeptide-immunoreactive neurons in the rat occipital cortex: a combined immunohistochemical-autoradiographic study. J Comp Neurol. 1989;284:637–645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K02 MH070031-07/MH/NIMH NIH HHS/United States
- R01 NS044080-08/NS/NINDS NIH HHS/United States
- R01 NS044080/NS/NINDS NIH HHS/United States
- P01 NS048120/NS/NINDS NIH HHS/United States
- P01 NS048120-040002/NS/NINDS NIH HHS/United States
- R01 MH066912-07/MH/NIMH NIH HHS/United States
- K02 MH070031/MH/NIMH NIH HHS/United States
- R01 MH066912/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous