Regulation of synaptic vesicle recycling by complex formation between intersectin 1 and the clathrin adaptor complex AP2 - PubMed (original) (raw)
. 2010 Mar 2;107(9):4206-11.
doi: 10.1073/pnas.0911073107. Epub 2010 Feb 16.
Jelena Bacetic, Ardeschir Vahedi-Faridi, Kira Gromova, Anna Sundborger, Nikolay Tomlin, Georg Krainer, Olga Vorontsova, Johannes G Schäfer, Simen G Owe, Michael A Cousin, Wolfram Saenger, Oleg Shupliakov, Volker Haucke
Affiliations
- PMID: 20160082
- PMCID: PMC2840162
- DOI: 10.1073/pnas.0911073107
Regulation of synaptic vesicle recycling by complex formation between intersectin 1 and the clathrin adaptor complex AP2
Arndt Pechstein et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Clathrin-mediated synaptic vesicle (SV) recycling involves the spatiotemporally controlled assembly of clathrin coat components at phosphatidylinositiol (4, 5)-bisphosphate [PI(4,5)P(2)]-enriched membrane sites within the periactive zone. Such spatiotemporal control is needed to coordinate SV cargo sorting with clathrin/AP2 recruitment and to restrain membrane fission and synaptojanin-mediated uncoating until membrane deformation and clathrin coat assembly are completed. The molecular events underlying these control mechanisms are unknown. Here we show that the endocytic SH3 domain-containing accessory protein intersectin 1 scaffolds the endocytic process by directly associating with the clathrin adaptor AP2. Acute perturbation of the intersectin 1-AP2 interaction in lamprey synapses in situ inhibits the onset of SV recycling. Structurally, complex formation can be attributed to the direct association of hydrophobic peptides within the intersectin 1 SH3A-B linker region with the "side sites" of the AP2 alpha- and beta-appendage domains. AP2 appendage association of the SH3A-B linker region inhibits binding of the inositol phosphatase synaptojanin 1 to intersectin 1. These data identify the intersectin-AP2 complex as an important regulator of clathrin-mediated SV recycling in synapses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Antibodies targeting the linker region between SH3 domains A and B of intersectin 1, a pre- and postsynaptic endocytic protein, inhibit SV recycling. (A) Intersectin 1 accumulates at pre- and postsynaptic membrane sites. Depicted are quantifications of immunogold particles accumulated over nerve terminals and dendrites in mossy fiber terminals and recurrent Schaffer collateral synapses within the mouse hippocampus labeled with intersectin 1-specific antibodies. The difference between densities of gold particles in each synaptic compartment is not significantly different for both types of synapses (P > 0.05; n = 20 in each case; Student's t test). Bars depict mean densities of gold particles ± SEM. Background is below two particles per square micrometers in both cases. (B and C) Electron micrographs of reticulospinal synapses in an axon stimulated at 5 Hz for 20 min after injection of antibodies against the linker region between SH3 domains A and B of lamprey intersectin (LIS-linker). The vesicle cluster (SVC) is depleted, large membrane invaginations (curved arrows) and clathrin-coated intermediates are present. Small arrow, active zone. a, axoplasm. (D) Electron micrograph of an intact reticulospinal synapse after injection of LIS-linker antibodies kept at rest. Note the presence of a densely packed SV cluster at the active zone. (E) Electron micrograph of a reticulospinal synapse in an axon stimulated at 5 Hz for 20 min after injection of antibodies against the SH3 domains C to E of lamprey intersectin (LIS-CE). Note the accumulation of CCPs and the absence of membrane invaginations. (F) Electron micrograph of an intact reticulospinal synapse stimulated at 5 Hz for 20 min from a reticulospinal axon from the same preparation as in E. (G) A synapse from an axon microinjected with nonspecific IgG and stimulated at 5 Hz. (H) Bar graph showing a reduction in the number of SVs in LIS antibody injected synapses. LIS-CE antibodies cause a strong increase in the number of CCPs (I), whereas LIS-linker antibody-injected synapses display numerous membrane pockets and invaginations (see curvature index, CI) (J). Nonspecific IgGs did not induce significant changes in synaptic morphology when compared to noninjected control synapses (H–J). Depicted are the means ± SD (*, P < 0.05; **, P < 0.01; ***, P < 0.001; Student's t test). (Scale bar, A and B, 1 μm.)
Fig. 2.
Intersectin associates with AP2 via direct binding to the α- and β2-appendage domains of AP2. (A) Immunoprecipitation from detergent-extracted rat brain lysates using control or anti-intersectin 1 (ITSN1) antibodies coupled to beads. Samples were analyzed by SDS/PAGE and immunoblotting. 1% input, 1% of the total amount of rat brain extract (RBE) added to the assay. (B) Same as in A but using detergent extracted lamprey brains instead. 1% input, 1% of the total amount of lamprey brain extract (LBE) added to the assay. (C) Electron micrograph of CCPs from the periactive zone of a lamprey reticulospinal synapse stimulated at 5 Hz for 30 min and labeled with intersectin LIS-AC antibodies. Note the accumulation of gold particles on CCPs. (Scale bar, 0.2 μm.) (D) Multiple sequence alignment of intersectin 1 SH3A-SH3B. Putative AP2-binding peptides are highlighted in purple. (E) GST or GST-intersectin 1 fusion proteins were immobilized and incubated with RBE in the presence or absence of ATP. Samples were analyzed by SDS/PAGE and immunoblotting. (F) Schematic representation of the proteins used for pull downs. Putative AP2 binding motifs and corresponding mutants are labeled (compare also to D). (G) High-affinity binding of intersectin 1 linker to the AP2β appendage as determined by isothermal titration calorimetry (ITC) analysis. Intersectin 1-derived linker peptide was injected into α- or β2-appendage. Depicted are integrated, normalized, and dilution-corrected heats of reaction, Q, versus molar peptide/protein ratio, R. Heats of reaction obtained by injecting peptide into α- (circles) or β2-appendage (squares) were fitted by a one-site binding model (solid lines), yielding the given _K_D values.
Fig. 3.
The intersectin 1-derived WADF motif peptide accommodates the sandwich subdomain of the AP2α or AP2β appendages. (A) Ribbon diagram showing the binding site for the mouse intersectin 1-derived peptide (amino acids 840–851, blue) in complex with the α-appendage (gray). The peptide binds to an elongated pocket at the side site of the α-appendage sandwich subdomain. (B) Close-up view showing direct molecular contacts between the intersectin 1-derived peptide (blue) and the side site of the α-appendage. See text for details. (C) Ribbon diagram showing the binding site for the mouse intersectin 1-derived peptide (blue) in complex with the β2-appendage (gray). The peptide binds to an elongated pocket at the side site of the β2-appendage sandwich subdomain. (D) Close-up view showing direct molecular contacts between the intersectin 1-derived peptide (blue) and the side site of the β2-appendage. See text for details. (E) One-hundred microgram GST-fusion protein or GST immobilized on glutathione beads were incubated with 2 mg RBE. Samples were analyzed by SDS/PAGE and immunoblotting. ITSN1 binding was abolished in the side site mutant Y815A but unaffected by mutation of the top site of the β2-appendage. (F) In vitro binding assay: 50 μg GST-fusion protein or GST immobilized on glutathione beads were incubated with 10 μg His6-tagged ITSN1 linker. A side site mutant (Y815A) of the β2-appendage fails to bind to the ITSN1 linker.
Fig. 4.
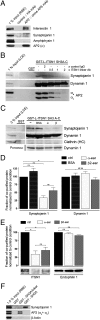
Association of intersectin 1 with AP2 impairs its binding to synaptojanin 1. (A) Top site-selective AP2 α-appendage antibodies (AP.6) coimmunoprecipitate intersectin but not synaptojanin, AP180, or amphiphysin from RBE. Samples were analyzed by SDS/PAGE and immunoblotting. 1% input, 1% of the total amount of RBE added to the assay. (B) Anti-intersectin linker domain antibodies inhibit complex formation of intersectin SH3A-C with AP2 and synaptojanin 1, but not with dynamin. GST-SH3A-C (LIS) or GST (100 μg) were used for affinity chromatography from lamprey spinal cord extracts (LCE) in the presence of the indicated molar ratios of IgGs targeting the intersectin 1 linker region or control IgGs. Samples were analyzed by SDS/PAGE and immunoblotting. 1% input, 1% of the total amount of LCE added to the assay. (C) Same as in B except that 1.5 mg purified α- or β2-appendage domains were used as competitors. Samples were analyzed by SDS/PAGE, staining with Ponceaus S (to visually illustrate dynamin 1 association), and immunoblotting for synaptojanin 1, dynamin 1, and clathrin heavy chain as a control. 3% input, 3% of the total amount of LCE added to the assay. (D) Quantification of the immunoblot signals for synaptojanin 1 and dynamin 1 seen in C (mean ± SEM; n = 3). (E) AP2 α- or β2-appendage domains inhibit complex formation between synaptojanin 1 and intersectin 1, but not endophilin A1. RBEs were subjected to immunoprecipitations with anti-synaptojanin 1-p145 antibodies in the presence or absence of AP2 α- or β2-appendage domains (500 μg). Depicted are the relative levels of intersectin 1 or endophilin A1 found in the immunoprecipitate normalized to the control condition (mean ± SEM; n = 2). Representative immunoblots for intersectin and endophilin are shown below the bar diagram. (F) GST-SH3A-linker, GST-SH3A, or GST (50 μg) were used for affinity chromatography from RBEs. Samples were analyzed by SDS/PAGE and immunoblotting for synaptojanin 1, AP2α, or β-actin. 1.5% input, 1.5% of the total amount of RBE added to the assay.
Similar articles
- Molecular mechanism and physiological functions of clathrin-mediated endocytosis.
McMahon HT, Boucrot E. McMahon HT, et al. Nat Rev Mol Cell Biol. 2011 Jul 22;12(8):517-33. doi: 10.1038/nrm3151. Nat Rev Mol Cell Biol. 2011. PMID: 21779028 Review. - Vesicle uncoating regulated by SH3-SH3 domain-mediated complex formation between endophilin and intersectin at synapses.
Pechstein A, Gerth F, Milosevic I, Jäpel M, Eichhorn-Grünig M, Vorontsova O, Bacetic J, Maritzen T, Shupliakov O, Freund C, Haucke V. Pechstein A, et al. EMBO Rep. 2015 Feb;16(2):232-9. doi: 10.15252/embr.201439260. Epub 2014 Dec 17. EMBO Rep. 2015. PMID: 25520322 Free PMC article. - Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin.
Gad H, Ringstad N, Löw P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, De Camilli P, Shupliakov O, Brodin L. Gad H, et al. Neuron. 2000 Aug;27(2):301-12. doi: 10.1016/s0896-6273(00)00038-6. Neuron. 2000. PMID: 10985350 - Role of epsin 1 in synaptic vesicle endocytosis.
Jakobsson J, Gad H, Andersson F, Löw P, Shupliakov O, Brodin L. Jakobsson J, et al. Proc Natl Acad Sci U S A. 2008 Apr 29;105(17):6445-50. doi: 10.1073/pnas.0710267105. Epub 2008 Apr 22. Proc Natl Acad Sci U S A. 2008. PMID: 18430801 Free PMC article. - Intersectin 1: a versatile actor in the synaptic vesicle cycle.
Pechstein A, Shupliakov O, Haucke V. Pechstein A, et al. Biochem Soc Trans. 2010 Feb;38(Pt 1):181-6. doi: 10.1042/BST0380181. Biochem Soc Trans. 2010. PMID: 20074056 Review.
Cited by
- The active zone protein Clarinet regulates synaptic sorting of ATG-9 and presynaptic autophagy.
Xuan Z, Yang S, Clark B, Hill SE, Manning L, Colón-Ramos DA. Xuan Z, et al. PLoS Biol. 2023 Apr 13;21(4):e3002030. doi: 10.1371/journal.pbio.3002030. eCollection 2023 Apr. PLoS Biol. 2023. PMID: 37053235 Free PMC article. - Eps15R and clathrin regulate EphB2-mediated cell repulsion.
Evergren E, Cobbe N, McMahon HT. Evergren E, et al. Traffic. 2018 Jan;19(1):44-57. doi: 10.1111/tra.12531. Epub 2017 Nov 6. Traffic. 2018. PMID: 28972287 Free PMC article. - Fast neurotransmitter release regulated by the endocytic scaffold intersectin.
Sakaba T, Kononenko NL, Bacetic J, Pechstein A, Schmoranzer J, Yao L, Barth H, Shupliakov O, Kobler O, Aktories K, Haucke V. Sakaba T, et al. Proc Natl Acad Sci U S A. 2013 May 14;110(20):8266-71. doi: 10.1073/pnas.1219234110. Epub 2013 Apr 30. Proc Natl Acad Sci U S A. 2013. PMID: 23633571 Free PMC article. - Dynamics and nanoscale organization of the postsynaptic endocytic zone at excitatory synapses.
Catsburg LA, Westra M, van Schaik AM, MacGillavry HD. Catsburg LA, et al. Elife. 2022 Jan 24;11:e74387. doi: 10.7554/eLife.74387. Elife. 2022. PMID: 35072626 Free PMC article. - Molecular mechanism and physiological functions of clathrin-mediated endocytosis.
McMahon HT, Boucrot E. McMahon HT, et al. Nat Rev Mol Cell Biol. 2011 Jul 22;12(8):517-33. doi: 10.1038/nrm3151. Nat Rev Mol Cell Biol. 2011. PMID: 21779028 Review.
References
- Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. - PubMed
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. - PubMed
- Shupliakov O. The synaptic vesicle cluster: a source of endocytic proteins during neurotransmitter release. Neuroscience. 2009;158:204–210. - PubMed
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. - PubMed
- Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous