Diacylglycerol kinase alpha mediates HGF-induced Rac activation and membrane ruffling by regulating atypical PKC and RhoGDI - PubMed (original) (raw)
Diacylglycerol kinase alpha mediates HGF-induced Rac activation and membrane ruffling by regulating atypical PKC and RhoGDI
Federica Chianale et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Diacylglycerol kinases (DGKs) convert diacylglycerol (DAG) into phosphatidic acid (PA), acting as molecular switches between DAG- and PA-mediated signaling. We previously showed that Src-dependent activation and plasma membrane recruitment of DGKalpha are required for growth-factor-induced cell migration and ruffling, through the control of Rac small-GTPase activation and plasma membrane localization. Herein we unveil a signaling pathway through which DGKalpha coordinates the localization of Rac. We show that upon hepatocyte growth-factor stimulation, DGKalpha, by producing PA, provides a key signal to recruit atypical PKCzeta/iota (aPKCzeta/iota) in complex with RhoGDI and Rac at ruffling sites of colony-growing epithelial cells. Then, DGKalpha-dependent activation of aPKCzeta/iota mediates the release of Rac from the inhibitory complex with RhoGDI, allowing its activation and leading to formation of membrane ruffles, which constitute essential requirements for cell migration. These findings highlight DGKalpha as the central element of a lipid signaling pathway linking tyrosine kinase growth-factor receptors to regulation of aPKCs and RhoGDI, and providing a positional signal regulating Rac association to the plasma membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
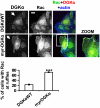
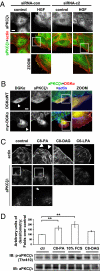
DGKα provides the signal directing Rac to the nascent ruffle. MDCK cells, transfected with either DGKαWT or myr-DGKα, were cultured overnight in the absence of serum, fixed, and stained for Rac (green), myc tag (red), and actin (blue). Arrows indicate DGKαWT- or myr-DGKα-transfected cells. (Scale bar, 10 μm.) C, 30 transfected cells, scored for the presence of Rac at ruffling sites. n = 6, with SEM; ***P = 0.0002.
Fig. 2.
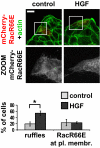
Rac plasma membrane targeting requires Rac/RhoGDI interaction. MDCK cells were transiently transfected with mCherry-RacR66E, treated with 10 ng/mL HGF for 15 min, fixed, and stained for actin (green). (Scale bar, 24 μm.) C, 30 transfected cells, scored for the presence of ruffles or RacR66E plasma membrane localization. n = 3, with SEM; *P = 0.0057.
Fig. 3.
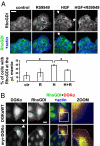
DGKα regulates RhoGDI targeting to the plasma membrane. (A) MDCK cells were stimulated with 10 ng/mL HGF for 5 min in the presence or absence of 1 μM R59949, fixed, and stained for RhoGDI (green) and actin (blue). Arrows indicate RhoGDI at membrane ruffles. (Scale bar, 24 μm.) C, 70 cells, scored for RhoGDI localization at ruffling sites. n = 3, with SEM; *P < 0.05. (B) MDCK cells, transfected with either DGKαWT or myr-DGKα, were cultured overnight in the absence of serum, fixed, and stained for RhoGDI (green), myc tag (red), and actin (blue). Arrowheads indicate transfected cells. (Scale bar, 10 μm.)
Fig. 4.
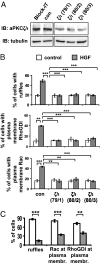
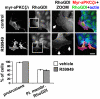
aPKCζ/ι mediates both HGF- and myr-DGKα-induced extension of membrane protrusions. (A) MDCK cells were transfected with three combinations of PKCζ (79, 80) and PKCι (1–3) specific siRNAs. Whole-cell lysates were analyzed for levels of aPKCζ/ι expression by western blot. (B) MDCK cells were transfected as in A, treated with 10 ng/mL HGF for 15 min, fixed, and stained for Rac or RhoGDI and actin. C, 110 cells, analyzed for the presence of ruffles and Rac or RhoGDI at the plasma membrane. n = 4 (Rac and RhoGDI), n = 8 (ruffles), with SEM; **P < 0.005, ***P < 0.0005. (C) MDCK cells were transiently transfected either with myr-DGKα alone or cotransfected with myr-DGKα and PKCζKW, cultured overnight in the absence of serum, fixed, and stained for myc and flag tags, actin, and Rac or RhoGDI. C, 20 transfected cells, scored for the presence of ruffles and Rac or RhoGDI at protrusion sites. n = 4, with SEM; **P < 0.001, ***P < 0.0001.
Fig. 5.
DGKα regulates aPKCζ/ι function. (A) MDCK cells were transfected either with control siRNA or DGKα siRNA c1 and c2, treated with 10 ng/mL HGF for 15 min, fixed, and stained for aPKCζ/ι (green) and actin (red). (Scale bar, 10 μm.) (B) MDCK cells, transfected either with DGKαWT or myr-DGKα, were cultured overnight in the absence of serum, fixed, and stained for aPKCζ/ι (green), myc tag (red), and actin (blue). Arrowheads indicate transfected cells. (Scale bar, 10 μm.) (C) MDCK cells were stimulated with either 250 μM C8-PA, C8-DAG, or C6-LPA or left untreated, and fixed and stained for actin (red) and aPKCζ/ι (green). Arrowheads indicate cortical actin rearrangements, while the arrow indicates aPKCζ/ι membrane localization. (Scale bar, 19 μm.) (D) MDCK cells were stimulated with either 250 μM C8-PA, 10% FCS medium (as positive control), 250 μM C8-DAG, or left untreated. Whole-cell lysates were analyzed by western blot and the intensity of phospho-aPKCζ/ι bands was quantified by densitometry. For each condition, nine replicate points were performed in four independent experiments. The densitometry of each band was normalized as the percentage of the densitometry mean of control points in the same experiment, and is shown in the histogram, with SEM; **P < 0.005. A representative picture is shown.
Fig. 6.
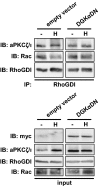
DGKα regulates Rac/RhoGDI complex dissociation. MDCK/empty vector or MDCK/DGKα-DN cells were stimulated with 50 ng/mL HGF for 15 min. Cell lysates were immunoprecipitated for RhoGDI and analyzed by western blot.
Fig. 7.
DGKα does not affect myr-PKCζ-induced events. MDCK cells were transiently transfected with myr-PKCζ, grown overnight in the absence of serum, and treated with 1 μM R59949 for 1 h. Cells were fixed and stained for flag tag (red), RhoGDI (green), and actin (blue). Arrows indicate RhoGDI staining at protrusion sites. (Scale bar, 24 μm.) C, 30 transfected cells, scored for the presence of protrusions and RhoGDI at protrusion sites. n = 3, with SEM.
Fig. 8.
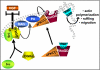
Model proposed for Rac-localized activation at the leading edge upon growth-factor stimulation. Upon growth-factor stimulation, DGKα is activated in a Src-dependent manner and recruited to the plasma membrane. The production of PA is the crucial signal to direct the recruitment of aPKCζ/ι, in complex with RhoGDI and Rac. PKCζ/ι, in turn, mediates the dissociation of Rac from the inhibitory complex with RhoGDI, which may become prone to activation by a RacGEF.
Similar articles
- Diacylglycerol kinase-alpha mediates hepatocyte growth factor-induced epithelial cell scatter by regulating Rac activation and membrane ruffling.
Chianale F, Cutrupi S, Rainero E, Baldanzi G, Porporato PE, Traini S, Filigheddu N, Gnocchi VF, Santoro MM, Parolini O, van Blitterswijk WJ, Sinigaglia F, Graziani A. Chianale F, et al. Mol Biol Cell. 2007 Dec;18(12):4859-71. doi: 10.1091/mbc.e07-02-0177. Epub 2007 Sep 26. Mol Biol Cell. 2007. PMID: 17898083 Free PMC article. - Diacylglycerol kinase zeta regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI.
Abramovici H, Mojtabaie P, Parks RJ, Zhong XP, Koretzky GA, Topham MK, Gee SH. Abramovici H, et al. Mol Biol Cell. 2009 Apr;20(7):2049-59. doi: 10.1091/mbc.e07-12-1248. Epub 2009 Feb 11. Mol Biol Cell. 2009. PMID: 19211846 Free PMC article. - The diacylglycerol kinase α/atypical PKC/β1 integrin pathway in SDF-1α mammary carcinoma invasiveness.
Rainero E, Cianflone C, Porporato PE, Chianale F, Malacarne V, Bettio V, Ruffo E, Ferrara M, Benecchia F, Capello D, Paster W, Locatelli I, Bertoni A, Filigheddu N, Sinigaglia F, Norman JC, Baldanzi G, Graziani A. Rainero E, et al. PLoS One. 2014 Jun 2;9(6):e97144. doi: 10.1371/journal.pone.0097144. eCollection 2014. PLoS One. 2014. PMID: 24887021 Free PMC article. - Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling.
Olofsson B. Olofsson B. Cell Signal. 1999 Aug;11(8):545-54. doi: 10.1016/s0898-6568(98)00063-1. Cell Signal. 1999. PMID: 10433515 Review. - Phosphatidic acid signaling regulation of Ras superfamily of small guanosine triphosphatases.
Zhang Y, Du G. Zhang Y, et al. Biochim Biophys Acta. 2009 Sep;1791(9):850-5. doi: 10.1016/j.bbalip.2009.05.013. Epub 2009 Jun 21. Biochim Biophys Acta. 2009. PMID: 19540930 Free PMC article. Review.
Cited by
- Interplay Between SNX27 and DAG Metabolism in the Control of Trafficking and Signaling at the IS.
González-Mancha N, Mérida I. González-Mancha N, et al. Int J Mol Sci. 2020 Jun 15;21(12):4254. doi: 10.3390/ijms21124254. Int J Mol Sci. 2020. PMID: 32549284 Free PMC article. Review. - "RACK"-ing up the effectors: Receptor for activated C kinase acts downstream of Rac GTPase signaling in growth cone outgrowth.
Demarco RS, Lundquist EA. Demarco RS, et al. Small GTPases. 2011 Jan;2(1):47-50. doi: 10.4161/sgtp.2.1.15062. Small GTPases. 2011. PMID: 21686282 Free PMC article. No abstract available. - Nuclear envelope phosphatase 1-regulatory subunit 1 (formerly TMEM188) is the metazoan Spo7p ortholog and functions in the lipin activation pathway.
Han S, Bahmanyar S, Zhang P, Grishin N, Oegema K, Crooke R, Graham M, Reue K, Dixon JE, Goodman JM. Han S, et al. J Biol Chem. 2012 Jan 27;287(5):3123-37. doi: 10.1074/jbc.M111.324350. Epub 2011 Dec 1. J Biol Chem. 2012. PMID: 22134922 Free PMC article. - Characterization of Novel Molecular Mechanisms Favoring Rac1 Membrane Translocation.
Castro-Castro A, Muriel O, Del Pozo MA, Bustelo XR. Castro-Castro A, et al. PLoS One. 2016 Nov 11;11(11):e0166715. doi: 10.1371/journal.pone.0166715. eCollection 2016. PLoS One. 2016. PMID: 27835684 Free PMC article. - Cholecystokinin-mediated RhoGDI phosphorylation via PKCα promotes both RhoA and Rac1 signaling.
Sabbatini ME, Williams JA. Sabbatini ME, et al. PLoS One. 2013 Jun 11;8(6):e66029. doi: 10.1371/journal.pone.0066029. Print 2013. PLoS One. 2013. PMID: 23776598 Free PMC article.
References
- Webb D-J, Parsons J-T, Horwitz A-F. Adhesion assembly, disassembly and turnover in migrating cells: over and over and over again. Nat Cell Biol. 2002;4:97–100. - PubMed
- Del Pozo M-A, et al. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol. 2002;4:232–239. - PubMed
- Del Pozo M-A, Schwartz M-A. Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol. 2007;17:246–250. - PubMed
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005;7:270–27. - PubMed
- Carrasco S, Merida I. Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci. 2006;32:27–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous