Nuclear entry of activated MAPK is restricted in primary ovarian and mammary epithelial cells - PubMed (original) (raw)
Nuclear entry of activated MAPK is restricted in primary ovarian and mammary epithelial cells
Elizabeth R Smith et al. PLoS One. 2010.
Abstract
Background: The MAPK/ERK1/2 serine kinases are primary mediators of the Ras mitogenic signaling pathway. Phosphorylation by MEK activates MAPK/ERK in the cytoplasm, and phospho-ERK is thought to enter the nucleus readily to modulate transcription.
Principal findings: Here, however, we observe that in primary cultures of breast and ovarian epithelial cells, phosphorylation and activation of ERK1/2 are disassociated from nuclear translocalization and transcription of downstream targets, such as c-Fos, suggesting that nuclear translocation is limited in primary cells. Accordingly, in import assays in vitro, primary cells showed a lower import activity for ERK1/2 than cancer cells, in which activated MAPK readily translocated into the nucleus and activated c-Fos expression. Primary cells express lower levels of nuclear pore complex proteins and the nuclear transport factors, importin B1 and importin 7, which may explain the limiting ERK1/2 import found in primary cells. Additionally, reduction in expression of nucleoporin 153 by siRNA targeting reduced ERK1/2 nuclear activity in cancer cells.
Conclusion: ERK1/2 activation is dissociated from nuclear entry, which is a rate limiting step in primary cells and in vivo, and the restriction of nuclear entry is disrupted in transformed cells by the increased expression of nuclear pores and/or nuclear transport factors.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
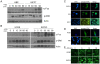
Figure 1. Nuclear localization of phospho-ERK is repressed in primary epithelial cells.
(A) Time course of ERK activation in HBE and MCF7 cells. Primary human breast epithelial (HBE) cells and MCF7 breast carcinoma cells were serum starved for 24 h to become quiescent (time 0), then stimulated with 15% FBS for 0–90 min, and phospho-ERK (p-ERK) and c-Fos expression, a downstream marker for nuclear phospho-ERK activity, were determined in cell lysates normalized for protein. (B) Time course of ERK and cFos activation in HOSE and SKOV3 cells. Primary human ovarian surface epithelial (HOSE) cells and SKOV3 ovarian carcinoma cells were grown as described for HBE and MCF7 cells, above. Expression of c-Fos and p-ERK was determined by immunoblotting. (C-E) Primary human breast and ovarian epithelial cells at passage 1 after isolation and breast and ovarian carcinoma cells were growth arrested in serum-free medium for 24 h, then stimulated with 15% serum, and analyzed for activated ERK (phospho-ERK). Cells for immunofluorescence were fixed and probed with an anti-phosphoERK1/2 monoclonal antibody (Sigma) followed by detection using a fluorescent secondary antibody. The nuclei were counterstained with DAPI and the two images merged using Adobe Photoshop, shown in the far right column. (C) Ovarian carcinoma SKOV3 and ES2 cells and MCF7 breast carcinoma cells show robust nuclear phospho-ERK localization after serum stimulation for 15 min. (D) In cultures of primary HOSE and HBE, phospho-ERK is localized primarily to the cytoplasm after serum stimulation for 15 min. (E) Primary cultures of early passage HOSE cells and SKOV3 were stimulated with FBS for the indicated times, then processed for immunofluorescence staining and confocal imaging. The results are representative of at least 3 experiments for each cell type.
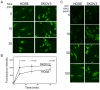
Figure 2. The kinetics of GFP-ERK2 nuclear import differs between primary ovarian epithelial and carcinoma cells.
(A) Time course of nuclear import. Digitonin-permeabilized primary HOSE or SKOV3 carcinoma cells were incubated with GFP-ERK2 (50 µg/ml or 0.8 µM) for the indicated times, then washed in ice cold buffer and fixed in 4% paraformaldehyde. (B) Fluorescence intensity of GFP-ERK2 was measured under identical settings for the experiments shown in A. The mean relative intensity is plotted +/− s.d. of more than 50 nuclei in duplicate, and significant differences were determined by Student's t-test, with the p-values denoted above the time point. (C) The uptake of GFP-ERK2 at concentrations ranging from 5 µg/ml (0.08 µM) to 100 µg/ml (1.6 µM) was measured after 15 min incubations with digitonin-permeabilized cells, as indicated above and in “Materials and Methods.”
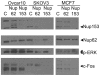
Figure 3. Immortalization and transformation increase transcription levels of nuclear import proteins.
(A,B) The expression of a panel of nuclear import factors was examined by Nanostring nCounter methodology for ovarian (A) and breast epithelial (B) cells. (A, Top Panel) Relative mRNA levels of importin 7 (Imp7) and importin B1 (ImpB1) in normal primary HOSE, HIO, and ovarian cancer cell lines. The numbers on the x-axis correspond to the cells listed to the right of the figure. (A, Middle Panel) Expression profiles for Nup153 and Nup214 for the set of cells listed, as in the top panel. (A, Bottom panel) The expression of a panel of nuclear import factors was examined by Nanostring nCounter methodology. The data represent the mean +/− s.d. for HOSE (n = 7), HIO (n = 1), and carcinoma (n = 7) cell cultures. Differences considered statistically significant (p<0.01), calculated using Student's t-test, are indicated by an “*”. Transcripts were examined for importin 7 (Imp7), importin-alpha6 (KPNA6), importin-beta1 (ImpB1), nucleoporin 153 (Nup153), nucleoporin 214 (Nup214), nucleoporin 62 (Nup62), nucleoporin 88 (Nup88), nuclear transport factor 2 (NUTF2), Ran binding protein 5 (RNBP5), and exportin 1 (XPO1). (B) Nanostring nCounter analyses were performed for 3 HBE, the immortalized MCF10 cells, and 4 breast carcinoma cell lines (MCF7, MDA-MB-231, MDA-MB-468, T47D), as in Figure 3A. (C) Immunoblot analyses were performed for Importin 7, Importin B, and NPC in primary HOSE and HBE cells, human immortalized ovarian epithelial (HIO) cells, MCF7 breast carcinoma cells, and a panel of ovarian carcinoma cells (ES2, UPN251, A1847, A2780, and Ovcar5). NPC proteins were detected with a pan-anti-NPC mouse monoclonal antibody (from Sigma). (D) Primary HOSE and HBE cells and SKOV3 and MCF7 carcinoma cells were fixed and stained for nuclear pores using a pan-anti-NPC mouse monoclonal antibody and an Alexa555-conjugated goat anti-mouse secondary antibody. All images were treated identically using Adobe Photoshop.
Figure 4. Suppressed expression of nucleoporins decreases c-Fos expression in carcinoma cells.
OVCAR-10 and SKOV3 ovarian and MCF7 breast cancer cells were transiently transfected with siRNA constructs to Nup153 and Nup62, as well as a control siRNA (from Santa Cruz). After 48 h, cells were serum-starved overnight, then stimulated with 20% FBS for 90 min, and lysed for immunoblot analysis. NPC proteins were detected with a mouse pan-anti-NPC monoclonal antibody.
Figure 5. NPC expression is upregulated in breast and ovarian carcinomas.
(A) Immunohistochemical analysis of NPC expression was undertaken on a tumor tissue microarray (TMA) containing normal breast epithelial tissue and tumor specimens, including DCIS (ductal carcinoma in situ), IDC (infiltrating ductal carcinoma), and ILC (infiltrating lobular carcinoma). A mouse pan-anti-NPC monoclonal antibody was used for immunostaining. (B) Immunohistochemical staining of NPC on ovarian TMA containing three ovarian serous carcinomas (OvCa) compared to a normal ovarian surface epithelium (left panel). Images were taken at 400× magnification.
Similar articles
- Stimulus-coupled spatial restriction of extracellular signal-regulated kinase 1/2 activity contributes to the specificity of signal-response pathways.
Whitehurst A, Cobb MH, White MA. Whitehurst A, et al. Mol Cell Biol. 2004 Dec;24(23):10145-50. doi: 10.1128/MCB.24.23.10145-10150.2004. Mol Cell Biol. 2004. PMID: 15542825 Free PMC article. - Cigarette smoke exposure reveals a novel role for the MEK/ERK1/2 MAPK pathway in regulation of CFTR.
Xu X, Balsiger R, Tyrrell J, Boyaka PN, Tarran R, Cormet-Boyaka E. Xu X, et al. Biochim Biophys Acta. 2015 Jun;1850(6):1224-32. doi: 10.1016/j.bbagen.2015.02.004. Epub 2015 Feb 16. Biochim Biophys Acta. 2015. PMID: 25697727 Free PMC article. - Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: Role of exogenous dietary fatty acids, p53-p21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-kappaB.
Menendez JA, Mehmi I, Atlas E, Colomer R, Lupu R. Menendez JA, et al. Int J Oncol. 2004 Mar;24(3):591-608. Int J Oncol. 2004. PMID: 14767544 - Nuclear extracellular signal-regulated kinase 1 and 2 translocation is mediated by casein kinase 2 and accelerated by autophosphorylation.
Plotnikov A, Chuderland D, Karamansha Y, Livnah O, Seger R. Plotnikov A, et al. Mol Cell Biol. 2011 Sep;31(17):3515-30. doi: 10.1128/MCB.05424-11. Epub 2011 Jul 5. Mol Cell Biol. 2011. PMID: 21730285 Free PMC article. Retracted.
Cited by
- Importin β1 regulates cell growth and survival during adult T cell leukemia/lymphoma therapy.
Ishikawa C, Senba M, Mori N. Ishikawa C, et al. Invest New Drugs. 2021 Apr;39(2):317-329. doi: 10.1007/s10637-020-01007-z. Epub 2020 Sep 21. Invest New Drugs. 2021. PMID: 32959166 - Casein kinase 2 sends extracellular signal-regulated kinase nuclear.
Whitmarsh AJ. Whitmarsh AJ. Mol Cell Biol. 2011 Sep;31(17):3512-4. doi: 10.1128/MCB.05916-11. Epub 2011 Jul 26. Mol Cell Biol. 2011. PMID: 21791609 Free PMC article. No abstract available. - The nuclear lamina regulates germline stem cell niche organization via modulation of EGFR signaling.
Chen H, Chen X, Zheng Y. Chen H, et al. Cell Stem Cell. 2013 Jul 3;13(1):73-86. doi: 10.1016/j.stem.2013.05.003. Cell Stem Cell. 2013. PMID: 23827710 Free PMC article. - KPNβ1 promotes palmitate-induced insulin resistance via NF-κB signaling in hepatocytes.
Wang S, Zhao Y, Xia N, Zhang W, Tang Z, Wang C, Zhu X, Cui S. Wang S, et al. J Physiol Biochem. 2015 Dec;71(4):763-72. doi: 10.1007/s13105-015-0440-x. Epub 2015 Oct 9. J Physiol Biochem. 2015. PMID: 26452501 - A tight balance of Karyopherin β1 expression is required in cervical cancer cells.
Carden S, van der Watt P, Chi A, Ajayi-Smith A, Hadley K, Leaner VD. Carden S, et al. BMC Cancer. 2018 Nov 16;18(1):1123. doi: 10.1186/s12885-018-5044-8. BMC Cancer. 2018. PMID: 30445944 Free PMC article.
References
- Gille H, Sharrocks AD, Shaw PE. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;359:414–424. - PubMed
- Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;65:663–675. - PubMed
- Corson LB, Yamanaka Y, Lai K-MV, Rossant J. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development. 2003;130:4527–4537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA079716/CA/NCI NIH HHS/United States
- R01CA099471/CA/NCI NIH HHS/United States
- R01CA79716/CA/NCI NIH HHS/United States
- R01CA75389/CA/NCI NIH HHS/United States
- R01 CA099471/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous