miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy - PubMed (original) (raw)
miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy
Kun Wang et al. J Biol Chem. 2010.
Abstract
Myocardial hypertrophy is frequently associated with poor clinical outcomes including the development of cardiac systolic and diastolic dysfunction and ultimately heart failure. To prevent cardiac hypertrophy and heart failure, it is necessary to identify and characterize molecules that may regulate the hypertrophic program. Our present study reveals that nuclear factor of activated T cells c3 (NFATc3) and myocardin constitute a hypertrophic pathway that can be targeted by miR-9. Our results show that myocardin expression is elevated in response to hypertrophic stimulation with isoproterenol and aldosterone. In exploring the molecular mechanism by which myocardin expression is elevated, we identified that NFATc3 can bind to the promoter region of myocardin and transcriptionally activate its expression. Knockdown of myocardin can attenuate hypertrophic responses triggered by NFATc3, suggesting that myocardin can be a downstream mediator of NFATc3 in the hypertrophic cascades. MicroRNAs are a class of small noncoding RNAs that mediate post-transcriptional gene silencing. Our data reveal that miR-9 can suppress myocardin expression. However, the hypertrophic stimulation with isoproterenol and aldosterone leads to a decrease in the expression levels of miR-9. Administration of miR-9 could attenuate cardiac hypertrophy and ameliorate cardiac function. Taken together, our data demonstrate that NFATc3 can promote myocardin expression, whereas miR-9 is able to suppress myocardin expression, thereby regulating cardiac hypertrophy.
Figures
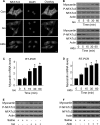
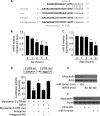
FIGURE 1.
Iso and Aldo induce an increase in myocardin expression levels and a decrease in the levels of phosphorylated NFATc3 in vitro and in vivo. A, Iso and Aldo induce NFATc3 redistributions from the cytoplasm to nuclei. The neonatal rat cardiomyocytes were treated with 10 μ
m
Iso or 1 μ
m
Aldo. 1 h after treatment the cells were collected for the analysis of NFATc3 by immunofluorescent staining. The cell nuclei were stained by 4′,6′-diamino-2-phenylindole (DAPI). Bar, 10 μm. B–D, the treatment with Iso and Aldo leads to an increase in myocardin expression levels and a decrease in the levels of phosphorylated NFATc3 (P-NFATc3). Cardiomyocytes were treated with 10 μ
m
Iso or 1 μ
m
Aldo. The cells were harvested at the indicated time for the analysis of myocardin protein levels, phosphorylated, and total NFATc3 levels by immunoblot (B) or myocardin mRNA levels by real time PCR (C and D). *, p < 0.05 versus control. E and F, Iso and Aldo treatment induces an elevation in myocardin levels and a reduction in the phosphorylated NFATc3 (P-NFATc3) in the animal model. C57BL/6 mice were infused with Iso or Aldo as described under “Experimental Procedures.” The levels of myocardin, P-NFATc3, and total NFATc3 in the hearts were analyzed by immunoblotting. A representative blot is shown. The data are expressed as the means ± S.E. of three independent experiments. Con, control.
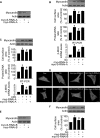
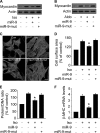
FIGURE 2.
Myocardin participates in mediating the hypertrophic signals of Iso and Aldo. A, knockdown of myocardin expression. Cardiomyocytes were infected with adenoviral myocardin-RNAi-A (myo-RNAi-A) or its scramble form (myo-S-RNAi-A) at a moi of 80. 48 h after infection the cells were harvested for the analysis of myocardin levels by immunoblot. B, knockdown of myocardin reduces hypertrophic responses induced by Iso. Cardiomyocytes were infected with adenoviral myocardin-RNAi-A or its scramble form (myocardin-S-RNAi-A) at a moi of 80. 24 h after infection the cells were treated with Iso. Analysis of myocardin levels by immunoblot was performed 1 h after Iso treatment (top panels). Hypertrophy was assessed by cell surface area measurement, protein/DNA ratio and β-MHC levels analyzed by qRT-PCR. *, p < 0.05 versus control; #, p < 0.05 versus Iso alone. C, myocardin is necessary for Aldo to induce hypertrophy. Cardiomyocytes were treated as described for B, except that Aldo was employed. *, p < 0.05 versus control; #, p < 0.05 versus Aldo alone. D, representative photos show sarcomere organization. Cardiomyocytes were treated as described for B or C. Bar, 20 μm. E, knockdown of myocardin expression. Cardiomyocytes were infected with adenoviral myocardin-RNAi-B (myo-RNAi-B) or its scramble form (myo-S-RNAi-B) at a moi of 100. 48 h after infection the cells were harvested for the analysis of myocardin levels by immunoblot. F, knockdown of myocardin reduces cell surface area induced by Iso. Cardiomyocytes were infected with adenoviral myocardin-RNAi-B or its scramble form (myocardin-S-RNAi-B) at a moi of 100. 24 h after infection cells were treated with Iso. Analysis of myocardin levels by immunoblot was performed 1 h after Iso treatment (top panels). Hypertrophy was assessed by cell surface area measurement (bottom panel). *, p < 0.05 versus control; #, p < 0.05 versus Iso alone. The data are expressed as the means ± S.E. of three independent experiments.
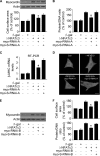
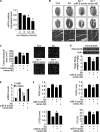
FIGURE 3.
NFATc3 transcriptionally targets myocardin. A and B, the constitutively active form of NFATc3 (ΔNFATc3) stimulates myocardin expression at protein and mRNA levels. Cardiomyocytes were infected with adenoviral β-gal orΔNFATc3 at a moi of 50. The cells were harvested at the indicated time for the analysis of myocardin protein levels by immunoblot (A) or myocardin mRNA levels by real time PCR (B). *, p < 0.05 versus control. C, myocardin promoter contains a potential NFATc3-binding site. The NFATc3 site is shown between −1734 and −1717 bp. The promoter of myocardin was synthesized and linked to luciferase (Luc) reporter gene. The mutations were introduced to the binding site (BS). D, chromatin immunoprecipitation (ChIP) analysis of in vivo NFATc3 binding to the promoter. Chromatin immunoprecipitation assay was performed using cardiomyocytes treated with or without 10 μ
m
Iso and 1 μ
m
Aldo. The anti-myocardin antibody was used as a negative control. E, NFATc3 activates myocardin promoter activity. Cardiomyocytes were infected with adenoviruses harboring β-gal or ΔNFATc3. 24 h after infection cells were transfected with the constructs of the empty vector (pGL-4.17), the wild type promoter (wt) or the promoter with mutations in the binding site (mBS), respectively. Firefly luciferase activities were normalized to Renilla luciferase activities. F and G, knockdown of endogenous NFATc3 inhibits the elevation of myocardin promoter activity induced by Iso or Aldo. Cardiomyocytes were infected with adenovirus harboring NFATc3 RNAi or its scramble form (NFATc3-S-RNAi) at a moi of 100. 24 h after infection cells were transfected with the construct of wild type promoter. Cardiomyocytes were treated with 10 μ
m
Iso (F) or 1 μ
m
Aldo (G). Firefly luciferase activities were normalized to Renilla luciferase activities. The data are expressed as the means ± S.E. of three independent experiments.
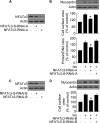
FIGURE 4.
Myocardin is able to convey the hypertrophic signal of NFATc3. A–D, cardiomyocytes were infected with adenoviral myocardin-RNAi-A (myo-RNAi-A) or its scramble form (myo-S-RNAi-A) at a moi of 80. 24 h after infection cells were infected with adenoviruses harboring β-gal or ΔNFATc3 at a moi of 50. Myocardin expression levels analyzed by immunoblot (top panels) and cell surface areas (bottom panel) are shown in A. Protein/DNA ratio analysis is shown in B. The expression levels of β-MHC analyzed by qRT-PCR were shown in C. *, p < 0.05 versus ΔNFATc3 alone. Representative photos show sarcomere organization (D). Bar, 20 μm. E and F, cardiomyocytes were infected with adenoviral myocardin-RNAi-B (myo-RNAi-B) or its scramble form (myo-S-RNAi-B) at a moi of 100. 24 h after infection cells were infected with adenoviruses harboring β-gal or ΔNFATc3 at a moi of 100. Myocardin expression levels analyzed by immunoblot are shown in E. Cell surface area measurement and protein/DNA ratio analysis are shown in F. *, p < 0.05 versus ΔNFATc3 alone. The data are expressed as the means ± S.E. of three independent experiments. Con, control.
FIGURE 5.
NFATc3 regulates myocardin expression in the hypertrophic pathway of Iso. A, knockdown of NFATc3 by RNAi-A. Cardiomyocytes were infected with adenoviral NFATc3 RNAi-A or its scramble form (NFATc3-S-RNAi-A). NFATc3 protein levels were analyzed by immunoblot 48 h after infection. B, knockdown of NFATc3 attenuates hypertrophic responses induced by Iso. Cardiomyocytes were infected with adenoviruses as described for A. 24 h after infection cells were exposed to 10 μ
m
Iso. Myocardin protein levels were analyzed by immunoblot. Hypertrophy was assessed by measuring cell surface area and protein/DNA ratio 48 h after treatment. *, p < 0.05 versus Iso alone. C, knockdown of NFATc3 by RNAi-B. Cardiomyocytes were infected with adenoviral NFATc3 RNAi-B or its scramble form (NFATc3-S-RNAi-B). NFATc3 protein levels were analyzed by immunoblot 48 h after infection. D, knockdown of NFATc3 attenuates hypertrophic responses induced by Iso. Cardiomyocytes were infected with adenoviruses as described for C. 24 h after infection cells were exposed to 10 μ
m
Iso. Myocardin protein levels were analyzed by immunoblot. Hypertrophy was assessed by measuring cell surface area 48 h after treatment. *, p < 0.05 versus Iso alone. The data are expressed as the means ± S.E. of three independent experiments.
FIGURE 6.
miR-9 inhibits myocardin expression. A, the miR-9 targeting sites in myocardin 3′-UTR are evolutionarily conserved in human, rat, and mouse. B, Iso induces a reduction of miR-9 levels. Cardiomyocytes were treated with 10 μ
m
Iso. The cells were harvested at the indicated time for the analysis of miR-9 levels. *, p < 0.05 versus control. C, Aldo induces a reduction of miR-9 levels. Cardiomyocytes were treated with 1 μ
m
Aldo. The cells were harvested at the indicated time for the analysis of miR-9 levels. *, p < 0.05 versus control. D, miR-9 suppresses myocardin translation. HEK293 cells were transfected with the luciferase constructs of the wild type myocardin-3′-UTR (Myocardin-3′-UTR-wt) or the mutated myocardin-3′-UTR (Myocardin-3′-UTR-mut), along with the expression plasmid for miR-9, miR-9 antagomir, or the antagomir negative control (Antagomir-NC). *, p < 0.05 versus myocardin-3′-UTR-wt; #, p < 0.05 versus myocardin-3′-UTR-wt plus miR-9. E, miR-9 suppresses the expression of myocardin in the cellular model. Cardiomyocytes were infected with adenoviral miR-9 or the mutated miR-9 (miR-9-mut) at the indicated moi. Myocardin expression was analyzed by immunoblot 48 h after infection. F, miR-9 suppresses the expression of myocardin in the animal model. Adult male C57BL/6 mice (8 weeks old) were infused with miR-9 mimic or the mimic negative control (mimic-NC) (30 mg/kg) as described under “Experimental Procedures.” Myocardin expression levels were analyzed by immunoblot 3 days after infusion. The data are expressed as the means ± S.E. of three independent experiments.
FIGURE 7.
miR-9 inhibits hypertrophic responses in the cellular model. A, miR-9 inhibits myocardin expression in cells treated with Iso. Cardiomyocytes were infected with the adenoviral miR-9 or the mutated miR-9 (miR-9-mut) at a moi of 100. 24 h after infection, the cells were treated with 10 μ
m
Iso. Myocardin expression was analyzed by immunoblot. B, miR-9 inhibits myocardin expression in cells treated with Aldo. Cardiomyocytes were treated as described for A, except that 1 μ
m
Aldo was used. Myocardin expression was analyzed by immunoblot. C–F, miR-9 attenuates hypertrophic responses induced by Iso. Cardiomyocytes were treated as described for A. C, sarcomere organization. Bar, 20 μm. D, cell surface area measurement. E, protein/DNA ratio. F, β-MHC expression levels. *, p < 0.05 versus Iso alone. The data are expressed as the means ± S.E. of three independent experiments.
FIGURE 8.
miR-9 inhibits cardiac hypertrophy in the animal model. A, Iso induces a reduction of miR-9 levels in the hearts. Adult male C57BL/6 mice (8 weeks old) were infused with Iso (30 mg/kg). The expression levels of miR-9 were determined with qRT-PCR. *, p < 0.05 versus control. B–E, miR-9 mimic inhibits cardiac hypertrophy. Adult male C57BL/6 mice (8 weeks old) were infused with Iso (30 mg/kg/day), along with miR-9 mimic or the mimic negative control (mimic-NC) (30 mg/kg) as described under “Experimental Procedures.” B, histological sections of hearts, gross hearts (top row; bar, 2 mm), heart sections stained with hematoxylin and eosin in the middle row (bar, 2 mm) and bottom row (bar, 20 μm). C, cross-sectional areas analyzed by staining with fluorescein isothiocyanate-conjugated wheat germ agglutinin. *, p < 0.05 versus Iso alone. D, the protein levels of myocardin in the hearts (top panels) and the ratio of heart/body weight (bottom panel). *, p < 0.05 versus Iso alone. E, the expression levels of atrial natriuretic peptide and β-MHC. *, p < 0.05 versus Iso alone. F, echocardiographic assessment of cardiac dimensions and function. Echocardiography was performed as described under “Experimental Procedures.” The mice were treated as described for B. Diastolic interventricular septal thickness (IVSd), diastolic posterior wall thickness (PWd), systolic left ventricular internal diameters (LVIDs), and fractional shortening of left ventricular diameter (FS). *, p < 0.05 versus Iso alone. The values represent means ± S.E. (n = 5–6). Con, control.
FIGURE 9.
Schematic model of cardiac hypertrophy regulated by miR-9. Iso or Aldo can induce a decrease in the levels of phosphorylated form of NFATc3 and miR-9, thereby leading to the elevation of myocardin that initiates the hypertrophic program. Administration of miR-9 mimic is able to attenuate cardiac hypertrophy.
Similar articles
- miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy.
Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li PF. Lin Z, et al. Proc Natl Acad Sci U S A. 2009 Jul 21;106(29):12103-8. doi: 10.1073/pnas.0811371106. Epub 2009 Jul 2. Proc Natl Acad Sci U S A. 2009. PMID: 19574461 Free PMC article. - LncRNA-Mhrt regulates cardiac hypertrophy by modulating the miR-145a-5p/KLF4/myocardin axis.
Xu Y, Luo Y, Liang C, Zhang T. Xu Y, et al. J Mol Cell Cardiol. 2020 Feb;139:47-61. doi: 10.1016/j.yjmcc.2019.12.013. Epub 2020 Jan 23. J Mol Cell Cardiol. 2020. PMID: 31982428 - NFATc3-dependent expression of miR-153-3p promotes mitochondrial fragmentation in cardiac hypertrophy by impairing mitofusin-1 expression.
Wang T, Zhai M, Xu S, Ponnusamy M, Huang Y, Liu CY, Wang M, Shan C, Shan PP, Gao XQ, Wang K, Chen XZ, Liu J, Xie JY, Zhang DY, Zhou LY, Wang K. Wang T, et al. Theranostics. 2020 Jan 1;10(2):553-566. doi: 10.7150/thno.37181. eCollection 2020. Theranostics. 2020. PMID: 31903137 Free PMC article. - MiR-466h-5p induces expression of myocardin with complementary promoter sequences.
Luo Y, Liang C, Xu Y, Zhang T. Luo Y, et al. Biochem Biophys Res Commun. 2019 Jun 18;514(1):187-193. doi: 10.1016/j.bbrc.2019.04.133. Epub 2019 Apr 25. Biochem Biophys Res Commun. 2019. PMID: 31029421 - MicroRNAs in cardiac disease.
Dorn GW 2nd. Dorn GW 2nd. Transl Res. 2011 Apr;157(4):226-35. doi: 10.1016/j.trsl.2010.12.013. Epub 2011 Jan 22. Transl Res. 2011. PMID: 21420033 Free PMC article. Review.
Cited by
- Resveratrol Ameliorates Cardiac Hypertrophy by Down-regulation of miR-155 Through Activation of Breast Cancer Type 1 Susceptibility Protein.
Fan Y, Liu L, Fang K, Huang T, Wan L, Liu Y, Zhang S, Yan D, Li G, Gao Y, Lv Y, Chen Y, Tu Y. Fan Y, et al. J Am Heart Assoc. 2016 Apr 22;5(4):e002648. doi: 10.1161/JAHA.115.002648. J Am Heart Assoc. 2016. PMID: 27107135 Free PMC article. - miR-206 represses hypertrophy of myogenic cells but not muscle fibers via inhibition of HDAC4.
Winbanks CE, Beyer C, Hagg A, Qian H, Sepulveda PV, Gregorevic P. Winbanks CE, et al. PLoS One. 2013 Sep 2;8(9):e73589. doi: 10.1371/journal.pone.0073589. eCollection 2013. PLoS One. 2013. PMID: 24023888 Free PMC article. - MicroRNA-19a/b-3p protect the heart from hypertension-induced pathological cardiac hypertrophy through PDE5A.
Liu K, Hao Q, Wei J, Li GH, Wu Y, Zhao YF. Liu K, et al. J Hypertens. 2018 Sep;36(9):1847-1857. doi: 10.1097/HJH.0000000000001769. J Hypertens. 2018. PMID: 29664809 Free PMC article. - Claudin-14 regulates renal Ca⁺⁺ transport in response to CaSR signalling via a novel microRNA pathway.
Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Gong Y, et al. EMBO J. 2012 Apr 18;31(8):1999-2012. doi: 10.1038/emboj.2012.49. Epub 2012 Feb 28. EMBO J. 2012. PMID: 22373575 Free PMC article. - Noncoding RNAs in Cardiac Hypertrophy.
Li Y, Liang Y, Zhu Y, Zhang Y, Bei Y. Li Y, et al. J Cardiovasc Transl Res. 2018 Dec;11(6):439-449. doi: 10.1007/s12265-018-9797-x. Epub 2018 Aug 31. J Cardiovasc Transl Res. 2018. PMID: 30171598 Review.
References
- Sugden P. H. (2003) Circ. Res. 93, 1179–1192 - PubMed
- Kass D. A., Bronzwaer J. G., Paulus W. J. (2004) Circ. Res. 94, 1533–1542 - PubMed
- Hardt S. E., Sadoshima J. (2002) Circ. Res. 90, 1055–1063 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous