Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice - PubMed (original) (raw)
. 2010 Mar;120(3):694-705.
doi: 10.1172/JCI40283. Epub 2010 Feb 22.
Affiliations
- PMID: 20179352
- PMCID: PMC2827954
- DOI: 10.1172/JCI40283
Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice
Mitomu Kioi et al. J Clin Invest. 2010 Mar.
Abstract
Despite the high doses of radiation delivered in the treatment of patients with glioblastoma multiforme (GBM), the tumors invariably recur within the irradiation field, resulting in a low cure rate. Understanding the mechanism of such recurrence is therefore important. Here we have shown in an intracranial GBM xenograft model that irradiation induces recruitment of bone marrow-derived cells (BMDCs) into the tumors, restoring the radiation-damaged vasculature by vasculogenesis and thereby allowing the growth of surviving tumor cells. BMDC influx was initiated by induction of HIF-1 in the irradiated tumors, and blocking this influx prevented tumor recurrence. Previous studies have indicated that BMDCs are recruited to tumors in part through the interaction between the HIF-1-dependent stromal cell-derived factor-1 (SDF-1) and its receptor, CXCR4. Pharmacologic inhibition of HIF-1 or of the SDF-1/CXCR4 interaction prevented the influx of BMDCs, primarily CD11b+ myelomonocytes, and the postirradiation development of functional tumor vasculature, resulting in abrogation of tumor regrowth. Similar results were found using neutralizing antibodies against CXCR4. Our data therefore suggest a novel approach for the treatment of GBM: in addition to radiotherapy, the vasculogenesis pathway needs to be blocked, and this can be accomplished using the clinically approved drug AMD3100, a small molecule inhibitor of SDF-1/CXCR4 interactions.
Figures
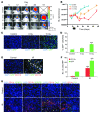
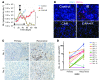
Figure 1. Irradiation promotes homing of BM-derived CD11b+ myeloid monocytic cells into GBM.
(A) Representative bioluminescent images of mouse heads with or without fractionated irradiation. Firefly luciferase–transduced U251 (U251/pFB-Luc) tumors were implanted i.c. and were irradiated from day 11 to day 15. 5 × 2 Gy, 2 Gy irradiation 5 times daily. (B) Tumor radioresponse was measured by BLI (n = 5 per group). Error bars indicate SD. (C) Radiation induced the influx of BMDCs into the U251 i.c. tumor. GFP-BM–transplanted nude mice received whole brain irradiation at 0, 8, or 15 Gy on day 22. Scale bar: 50 μm. (D) Quantification of GFP-BM influx into the tumor. Error bars indicate SEM. ***P < 0.001 versus control. (E) Representative images of IHC for GFP-BM cells and CD11b+ myeloid monocytic cells in U251 i.c. tumor before and after irradiation. Arrowheads indicate CD11b+ GFP-BM cells. Scale bar: 50 μm. (F) Quantification of CD11b and GFP-BM influx into the tumor. Error bars indicate SEM. ***P < 0.001. (G) Characterization of CD11b+ infiltrating cells into tumor after irradiation. Representative images of IHC staining for DAPI (blue), CD11b (red), and other markers (VEGFR1, VEGFR2, Tie-2, or Gr-1; green) in i.c. tumors after irradiation. Scale bar: 50 μm. IR, irradiation.
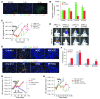
Figure 2. HIF-1 activity is increased in irradiated tumors and is necessary for influx of BMDCs and tumor recurrence after irradiation.
(A) Representative images of IHC staining for hypoxia (pimonidazole [pimo]) and functional vessels (lectin perfusion [perf]) in i.c. tumors after irradiation. Scale bar: 50 μm. (B) Quantification of hypoxic areas and vessels in the tumors of A. Error bars indicate SEM. *P < 0.05, **P < 0.01 versus control. Note the log scale on the ordinate. (C) BLI of 5HRE-Luc or pFB-Luc U251 i.c. tumors after a single dose (arrowhead) of irradiation on day 32. The divergence of the 2 curves starting at about 2 weeks after irradiation indicates increased HIF-1 activity. Error bars indicate SD. (D) Effect of NSC-134754 on inhibition of HIF-1 activity in vivo. 5HRE-Luc or pFB-Luc U251 tumor–bearing mice were treated with 15 Gy of irradiation, followed by daily injection of NSC-134754 for 3 weeks. Images were taken on the day of irradiation and 3 weeks later. (E) IHC staining for leukocyte (CD45) and monocyte (CD11b, top row) or macrophage (F4/80, bottom row) infiltration into i.c. tumors. Tumors were harvested on the day of irradiation for controls and 17 days after irradiation in treatment groups. Scale bar: 50 μm. (F) Quantification of CD11b+ and F4/80+ cell influx in tumors of E. Error bars indicate SEM. **P < 0.01, ***P < 0.001 versus control. (G) Growth curves of i.c. tumors treated with irradiation, NSC-134754, irradiation+NSC-134754, and control (n = 5 per group). Error bars indicate SD. *P < 0.05. (H) Growth curves of orthotopic U251/pSR (vector) and U251 HIF1KD1+4 tumors using BLI (n = 7). A whole brain irradiation was given on day 22. Error bars indicate SD. *P < 0.05.
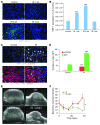
Figure 3. The interaction of SDF-1 and CXCR4 promotes tumor influx of BMDCs and restoration of tumor vasculature.
(A) Irradiation promotes the expression of SDF-1 in U251 i.c. tumor. Representative image of IHC staining for SDF-1. Scale bar: 50 μm. (B) Quantification of SDF-1 in the irradiated tumors. Error bars indicate SEM. ***P < 0.001 versus control. (C) Phosphorylation of CXCR4 on BMDCs in tumors was induced after irradiation. IHC staining for GFP-BM and pCXCR4 in U251 i.c. tumors 3 weeks after 15 Gy whole brain irradiation. Arrowheads indicate phospho-CXCR4 GFP-BM cells. Scale bar: 50 μm. (D) Quantification of pCXCR4 GFP-BM cells in U251 i.c. tumor after irradiation. Error bars indicate SEM. ***P < 0.001. (E) AMD3100 prevents the restoration of tumor blood flow (green) after irradiation. Representative ultrasound images from U251 s.c. tumors treated with 15 Gy irradiation, AMD3100, irradiation+AMD3100, and control. Scale bar: 1 mm. (F) Quantification of blood flow in tumor of E. Blood flow was reduced by irradiation but recovered by 3 weeks. AMD3100 plus IR prevents the recovery of tumor blood flow. Error bars indicate SD. *P < 0.05.
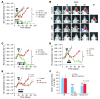
Figure 4. Therapeutic effect of blocking the interaction of SDF-1 with CXCR4 after whole brain irradiation.
(A) Growth curves of i.c. U251 early tumor model after fractionated irradiation (5 daily doses of 2 Gy starting on day 11 after transplantation). *P < 0.05. (B) BLI images after fractionated irradiation treated with or without AMD3100. (C and D) Growth curves of i.c. U251 advanced tumor model after a single dose of irradiation (15 Gy on day 22 after transplantation), treated with AMD3100 (21 day infusion; C, *P < 0.05) or with neutralizing anti-CXCR4 Ab (D, *P < 0.05), starting immediately after irradiation. (E) Growth curves of U251 i.c. tumor after 15 Gy irradiation, treated with DC101. Arrowheads indicate the treatment of DC101 (started immediately after irradiation and maintained for 21 days). (F) AMD3100 is more effective than DC101 in reducing tumor blood flow. Quantification of endothelial cells and functional vessels in U251 s.c. tumors after 15 Gy irradiation and combined with AMD3100 or DC101. Samples were taken 17 days after irradiation. Error bars indicate SEM. **P < 0.01, ***P < 0.001 versus IR.
Figure 5. CD11b+ cells are associated with GBM tumor recurrences in U251 i.c. tumors in mice and in patients.
(A) Growth of U251 i.c. tumor assessed by BLI. Tumor-bearing mice were treated with 15 Gy whole brain irradiation, carrageenan (CAR), or the combination of irradiation and CAR. The arrow indicates irradiation, and the bar indicates the period of CAR treatment. Error bars indicate SD. *P < 0.05. (B) IHC of U251 i.c. tumor of A for CD11b. The average of CD11b cells per HPF was 6.3 (control), 72.6 (IR), 2.6 (CAR), and 2.0 (CAR+IR). (C) IHC of GBM clinical samples staining for CD11b. (D) Significantly increased levels of CD11b+ cells in the recurrent human GBMs compared with the untreated tumors. Quantification of CD11b based on IHC with CD11b staining. Eight of twelve samples showed significant increases in CD11b+ cells in recurrent GBMs (P < 0.05). Error bars indicate SEM. Scale bars: 50 μm.
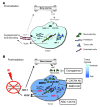
Figure 6. Model of the main contributions of BMDCs; and cytokines that promote restoration of tumor vasculature after irradiation.
(A) Prior to irradiation, tumor growth is governed largely by local angiogenesis. When local angiogenesis is inhibited by irradiation, growth of the tumor vasculature (essential for recurrence of the tumor) can only occur from circulating cells, of which BMDCs are an essential component. (B) After irradiation, the tumor becomes more hypoxic, and HIF-1 is increased as the tumor attempts to regrow. This induces SDF-1 and promotes the mobilization of CD11b+ monocytes from the BM and retention of these BMDCs into the tumor. SDF-1 is the key factor for the influx of BMDCs, as AMD3100, an inhibitor of CXCR4/SDF-1, and antibodies against CXCR4 block the recruitment and/or tumor retention of the BMDCs, inhibit restoration of the tumor vasculature, and prevent tumor recurrence. The various inhibitors and the points in the cycle at which they act are shown in boxes. Mϕ, macrophages.
Comment in
- Resisting arrest: a switch from angiogenesis to vasculogenesis in recurrent malignant gliomas.
Greenfield JP, Cobb WS, Lyden D. Greenfield JP, et al. J Clin Invest. 2010 Mar;120(3):663-7. doi: 10.1172/JCI42345. Epub 2010 Feb 22. J Clin Invest. 2010. PMID: 20179347 Free PMC article.
Similar articles
- Resisting arrest: a switch from angiogenesis to vasculogenesis in recurrent malignant gliomas.
Greenfield JP, Cobb WS, Lyden D. Greenfield JP, et al. J Clin Invest. 2010 Mar;120(3):663-7. doi: 10.1172/JCI42345. Epub 2010 Feb 22. J Clin Invest. 2010. PMID: 20179347 Free PMC article. - Recurrence of glioblastoma after radio-chemotherapy is associated with an angiogenic switch to the CXCL12-CXCR4 pathway.
Tabouret E, Tchoghandjian A, Denicolai E, Delfino C, Metellus P, Graillon T, Boucard C, Nanni I, Padovani L, Ouafik L, Figarella-Branger D, Chinot O. Tabouret E, et al. Oncotarget. 2015 May 10;6(13):11664-75. doi: 10.18632/oncotarget.3256. Oncotarget. 2015. PMID: 25860928 Free PMC article. - Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation.
Kozin SV, Kamoun WS, Huang Y, Dawson MR, Jain RK, Duda DG. Kozin SV, et al. Cancer Res. 2010 Jul 15;70(14):5679-85. doi: 10.1158/0008-5472.CAN-09-4446. Epub 2010 Jul 14. Cancer Res. 2010. PMID: 20631066 Free PMC article. - Inhibiting vasculogenesis after radiation: a new paradigm to improve local control by radiotherapy.
Martin BJ. Martin BJ. Semin Radiat Oncol. 2013 Oct;23(4):281-7. doi: 10.1016/j.semradonc.2013.05.002. Semin Radiat Oncol. 2013. PMID: 24012342 Free PMC article. Review. - Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy.
Brown JM. Brown JM. Br J Radiol. 2014 Mar;87(1035):20130686. doi: 10.1259/bjr.20130686. Br J Radiol. 2014. PMID: 24338942 Free PMC article. Review.
Cited by
- Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization.
Pombo Antunes AR, Scheyltjens I, Lodi F, Messiaen J, Antoranz A, Duerinck J, Kancheva D, Martens L, De Vlaminck K, Van Hove H, Kjølner Hansen SS, Bosisio FM, Van der Borght K, De Vleeschouwer S, Sciot R, Bouwens L, Verfaillie M, Vandamme N, Vandenbroucke RE, De Wever O, Saeys Y, Guilliams M, Gysemans C, Neyns B, De Smet F, Lambrechts D, Van Ginderachter JA, Movahedi K. Pombo Antunes AR, et al. Nat Neurosci. 2021 Apr;24(4):595-610. doi: 10.1038/s41593-020-00789-y. Epub 2021 Mar 29. Nat Neurosci. 2021. PMID: 33782623 - The impact of the myeloid response to radiation therapy.
Gough MJ, Young K, Crittenden M. Gough MJ, et al. Clin Dev Immunol. 2013;2013:281958. doi: 10.1155/2013/281958. Epub 2013 Apr 7. Clin Dev Immunol. 2013. PMID: 23653658 Free PMC article. Review. - The HIF-pathway inhibitor NSC-134754 induces metabolic changes and anti-tumour activity while maintaining vascular function.
Baker LC, Boult JK, Walker-Samuel S, Chung YL, Jamin Y, Ashcroft M, Robinson SP. Baker LC, et al. Br J Cancer. 2012 May 8;106(10):1638-47. doi: 10.1038/bjc.2012.131. Epub 2012 Apr 12. Br J Cancer. 2012. PMID: 22498643 Free PMC article. - Organoids as Complex In Vitro Models for Studying Radiation-Induced Cell Recruitment.
Hacker BC, Rafat M. Hacker BC, et al. Cell Mol Bioeng. 2020 Jun 15;13(4):341-357. doi: 10.1007/s12195-020-00625-0. eCollection 2020 Aug. Cell Mol Bioeng. 2020. PMID: 32952734 Free PMC article. Review. - High-resolution in-vivo analysis of normal brain response to cranial irradiation.
Burrell K, Hill RP, Zadeh G. Burrell K, et al. PLoS One. 2012;7(6):e38366. doi: 10.1371/journal.pone.0038366. Epub 2012 Jun 4. PLoS One. 2012. PMID: 22675549 Free PMC article.
References
- Souhami L, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60(3):853–860. - PubMed
- Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30(9):907–911. - PubMed
- Liang BC, Thornton AF, Jr, Sandler HM, Greenberg HS. Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg. 1991;75(4):559–563. - PubMed
- Sneed PK, et al. Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys. 1994;29(4):719–727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials