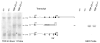Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis - PubMed (original) (raw)
Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis
Brian C Kraemer et al. Acta Neuropathol. 2010 Apr.
Abstract
Abnormal TDP-43 aggregation is a prominent feature in the neuropathology of amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration. Mutations in TARDBP, the gene encoding TDP-43, cause some cases of ALS. The normal function of TDP-43 remains incompletely understood. To better understand TDP-43 biology, we generated mutant mice carrying a genetrap disruption of Tardbp. Mice homozygous for loss of TDP-43 are not viable. TDP-43 deficient embryos die about day 7.5 of embryonic development thereby demonstrating that TDP-43 protein is essential for normal prenatal development and survival. However, heterozygous Tardbp mutant mice exhibit signs of motor disturbance and muscle weakness. Compared with wild type control littermates, Tardbp (+/-) animals have significantly decreased forelimb grip strength and display deficits in a standard inverted grid test despite no evidence of pathologic changes in motor neurons. Thus, TDP-43 is essential for viability, and mild reduction in TDP-43 function is sufficient to cause motor deficits without degeneration of motor neurons.
Figures
Fig. 1
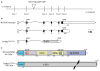
Genetrap targeting of the Tardbp gene. a The genetrap insertion site on mouse chromosome 4 is depicted. Vertical bars are exons, intervening lines represent introns. The position on chromo-some 4 is indicated. b The predicted mRNAs encoding TDP-43 and the truncated genetrap product are indicated. c The protein domain structure of normal TDP-43 and the TDP-43/beta-geo fusion protein are indicated
Fig. 2
Expression of Tardbp mRNA. Northern blot of brain mRNA purified from Tardbp+/+ and Tardbp+/− animals. The normal short and long 3′ UTR forms of the TDP-43 encoding message are indicated (7.7 and 2.7 kb products). The genetrap truncated mRNA is the intermediate 5.4 Kb band only present in Tardbp+/− animals. The exon 1 targeted radio labeled probe is indicated (left panel). Genetrap specific probe binds beta-geo coding sequence (right panel)
Fig. 3
Expression of TDP-43 protein in various tissues. Immunoblotting of brain, heart, kidney, liver, lung, muscle, and spleen tissue protein extracts. Duplicate blots were probed with a pan-TDP-43 specific polyclonal antibodies and b beta-geo specific antibody. Arrow indicates the TDP-43(1–65)/beta-geo fusion protein. Arrowhead indicates the major 43 kDa brain isoform of TDP-43 protein. c beta-Actin specific antibody
Fig. 4
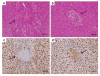
Degeneration of in utero embryos lacking TDP-43 protein. a H&E stained section of E7.5 embryo (arrow) undergoing necrosis; the surrounding tissue is uterine deciduas. b H&E stained section of a normal E7.5 embryo surrounded by uterine decidual tissue. c TDP immunohistochemistry demonstrating lack of TDP staining in the degenerating E7.5 embryo (arrow) surrounded by TDP positive uterine decidual tissue. d TDP immunohistochemistry normal E7.5 embryo (arrow) showing positive staining in the embryo as well as in the surrounding uterine decidual tissue
Fig. 5
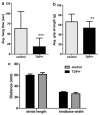
Tardbp+/− animals display deficits in grip strength relative to littermate controls. a Tardbp+/− animals (n = 18) hung on an inverted wire grid for significantly less time than control animals (n = 22) and b also exhibit lower maximum forelimb grip strength. Individual scores are the average of three trials, and the error bars represent standard deviation (**_p_= 0.01; ***_p_= 0.0002) c A subset of these animals was also examined for differences in gait, as measured by average stride length and the width between hindpaws while walking. No significant difference between the genotypes was detected
Similar articles
- Mutant TDP-43 Causes Early-Stage Dose-Dependent Motor Neuron Degeneration in a TARDBP Knockin Mouse Model of ALS.
Ebstein SY, Yagudayeva I, Shneider NA. Ebstein SY, et al. Cell Rep. 2019 Jan 8;26(2):364-373.e4. doi: 10.1016/j.celrep.2018.12.045. Cell Rep. 2019. PMID: 30625319 - Short-term suppression of A315T mutant human TDP-43 expression improves functional deficits in a novel inducible transgenic mouse model of FTLD-TDP and ALS.
Ke YD, van Hummel A, Stevens CH, Gladbach A, Ippati S, Bi M, Lee WS, Krüger S, van der Hoven J, Volkerling A, Bongers A, Halliday G, Haass NK, Kiernan M, Delerue F, Ittner LM. Ke YD, et al. Acta Neuropathol. 2015 Nov;130(5):661-78. doi: 10.1007/s00401-015-1486-0. Epub 2015 Oct 5. Acta Neuropathol. 2015. PMID: 26437864 - Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo.
Kabashi E, Lin L, Tradewell ML, Dion PA, Bercier V, Bourgouin P, Rochefort D, Bel Hadj S, Durham HD, Vande Velde C, Rouleau GA, Drapeau P. Kabashi E, et al. Hum Mol Genet. 2010 Feb 15;19(4):671-83. doi: 10.1093/hmg/ddp534. Epub 2009 Dec 3. Hum Mol Genet. 2010. PMID: 19959528 - Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis.
Pesiridis GS, Lee VM, Trojanowski JQ. Pesiridis GS, et al. Hum Mol Genet. 2009 Oct 15;18(R2):R156-62. doi: 10.1093/hmg/ddp303. Hum Mol Genet. 2009. PMID: 19808791 Free PMC article. Review. - The molecular link between inefficient GluA2 Q/R site-RNA editing and TDP-43 pathology in motor neurons of sporadic amyotrophic lateral sclerosis patients.
Yamashita T, Kwak S. Yamashita T, et al. Brain Res. 2014 Oct 10;1584:28-38. doi: 10.1016/j.brainres.2013.12.011. Epub 2013 Dec 16. Brain Res. 2014. PMID: 24355598 Review.
Cited by
- TDP-43 prevents endogenous RNAs from triggering a lethal RIG-I-dependent interferon response.
Dunker W, Ye X, Zhao Y, Liu L, Richardson A, Karijolich J. Dunker W, et al. Cell Rep. 2021 Apr 13;35(2):108976. doi: 10.1016/j.celrep.2021.108976. Cell Rep. 2021. PMID: 33852834 Free PMC article. - Rodent models of TDP-43: recent advances.
Tsao W, Jeong YH, Lin S, Ling J, Price DL, Chiang PM, Wong PC. Tsao W, et al. Brain Res. 2012 Jun 26;1462:26-39. doi: 10.1016/j.brainres.2012.04.031. Epub 2012 May 1. Brain Res. 2012. PMID: 22608070 Free PMC article. Review. - Optogenetic TDP-43 nucleation induces persistent insoluble species and progressive motor dysfunction in vivo.
Otte CG, Fortuna TR, Mann JR, Gleixner AM, Ramesh N, Pyles NJ, Pandey UB, Donnelly CJ. Otte CG, et al. Neurobiol Dis. 2020 Dec;146:105078. doi: 10.1016/j.nbd.2020.105078. Epub 2020 Sep 12. Neurobiol Dis. 2020. PMID: 32927062 Free PMC article. - ALS Genetics: Gains, Losses, and Implications for Future Therapies.
Kim G, Gautier O, Tassoni-Tsuchida E, Ma XR, Gitler AD. Kim G, et al. Neuron. 2020 Dec 9;108(5):822-842. doi: 10.1016/j.neuron.2020.08.022. Epub 2020 Sep 14. Neuron. 2020. PMID: 32931756 Free PMC article. Review. - Neuronal models of TDP-43 proteinopathy display reduced axonal translation, increased oxidative stress, and defective exocytosis.
Pisciottani A, Croci L, Lauria F, Marullo C, Savino E, Ambrosi A, Podini P, Marchioretto M, Casoni F, Cremona O, Taverna S, Quattrini A, Cioni JM, Viero G, Codazzi F, Consalez GG. Pisciottani A, et al. Front Cell Neurosci. 2023 Nov 13;17:1253543. doi: 10.3389/fncel.2023.1253543. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38026702 Free PMC article.
References
- Abhyankar MM, Urekar C, Reddi PP. A novel CpG-free vertebrate insulator silences the testis-specific SP-10 gene in somatic tissues: role for TDP-43 in insulator function. J Biol Chem. 2007;282:36143–36154. - PubMed
- Aubin JE, Liu F, Candeliere GA. Single-cell PCR methods for studying stem cells and progenitors. Methods Mol Biol. 2002;185:403–415. - PubMed
- Ayala YM, Pantano S, D'Ambrogio A, Buratti E, Brindisi A, Marchetti C, Romano M, Baralle FE. Human, Drosophila, and C. elegans TDP43: nucleic acid binding properties and splicing regulatory function. J Mol Biol. 2005;348:575–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG017586/AG/NIA NIH HHS/United States
- R01 NS064131/NS/NINDS NIH HHS/United States
- AG17586/AG/NIA NIH HHS/United States
- R01 NS064131-01A1/NS/NINDS NIH HHS/United States
- NS064131/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous