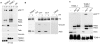Endocytosis of the anthrax toxin is mediated by clathrin, actin and unconventional adaptors - PubMed (original) (raw)
Endocytosis of the anthrax toxin is mediated by clathrin, actin and unconventional adaptors
Laurence Abrami et al. PLoS Pathog. 2010.
Abstract
The anthrax toxin is a tripartite toxin, where the two enzymatic subunits require the third subunit, the protective antigen (PA), to interact with cells and be escorted to their cytoplasmic targets. PA binds to cells via one of two receptors, TEM8 and CMG2. Interestingly, the toxin times and triggers its own endocytosis, in particular through the heptamerization of PA. Here we show that PA triggers the ubiquitination of its receptors in a beta-arrestin-dependent manner and that this step is required for clathrin-mediated endocytosis. In addition, we find that endocytosis is dependent on the heterotetrameric adaptor AP-1 but not the more conventional AP-2. Finally, we show that endocytosis of PA is strongly dependent on actin. Unexpectedly, actin was also found to be essential for efficient heptamerization of PA, but only when bound to one of its 2 receptors, TEM8, due to the active organization of TEM8 into actin-dependent domains. Endocytic pathways are highly modular systems. Here we identify some of the key players that allow efficient heptamerization of PA and subsequent ubiquitin-dependent, clathrin-mediated endocytosis of the anthrax toxin.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
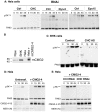
Figure 1. Anthrax toxin enter cells by clathrin mediated endocytosis.
A: Hela cells were transfected 72 hrs with control siRNAs or siRNAs against Clathrin Heavy Chain (CHC), the dynamin 2 (Dyn-2) or Epidermal growth factor receptor pathway substrate 15 (Eps 15). Cells were incubated with 500 ng/ml PA63 for 1 hr at 4°C and different times at 37°C and cell extracts (40 µg of proteins) were analyzed by SDS-PAGE and western blotting to reveal PA monomer and SDS-resistant heptamer (pPA7mer). B: Cell extracts (40 µg of protein) from Raw, BHK and Hela–transfected or not with mouse CMG2-4–cells were analyzed by SDS-PAGE and Western blotting for the expression of CMG2. C: Stable BHK21-tTA/anti-CHC cells maintained in tetracycline (control cells) or not (CHC AS) were incubated with 500 ng/ml PA63 for 1 hr at 4°C and different times at 37°C and extracts (40 µg of proteins) were analyzed by SDS-PAGE and western blotting to reveal PA63 and SDS-resistant heptamer (pPA7mer). DE: Hela cells were transfected 72 hrs with human CMG2-V5 and with control siRNAs or siRNAs against CHC. Cells were then treated as in A. Cells extracts (40 µg of proteins) were analyzed by SDS-PAGE and western blotting to reveal the different forms of PA, CMG2-V5 and the N-terminus of the LF target MEK1 (MEK1 (N)).
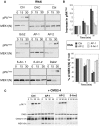
Figure 2. Endocytosis of PA is β-arrestin and AP-1 dependent.
A-B: Hela cells were transfected 72 hrs with control siRNAs or siRNAs against Clathrin Heavy Chain (CHC), the ubiquitin ligase E3 Cbl (Cbl), Grb2, AP-1, AP-2, β-arrestin-1 (β-Arr-1), β-arrestin-2 (β-Arr-2) or Dab2. The efficiency of siRNAs was analyzed on cell extracts (40 µg of proteins) by SDS-PAGE and western blotting (Fig. S1). Cells were incubated with 500 ng/ml PA63 and 100 ng/ml LF for 1 hour at 4°C and different times at 37°C and cell extracts (40 µg of proteins) were analyzed by SDS-PAGE and western blotting to reveal PA SDS-resistant heptamer (pPA7mer) and N-terminus of MEK1 (MEK1 (N)). B: Levels of pPA7mer and full length MEK1 were quantified using the Typhoon scanner and normalized to 1 and 100% respectively at time 0 (1 hour at 4°C). The plot represents the means of 4 independent experiments. Errors represent standard deviations. C: Hela cells were transfected 72 hours with CMG2-V5 and control siRNAs or siRNAs against AP-1, AP-2 or β-arrestin-2 (β-Arr-2). Cell extracts were subsequently analyzed by SDS-PAGE and western blotting to reveal the different forms of PA, CMG2-V5 and N-terminus of MEK1 (MEK1 (N)).
Figure 3. Endocytosis of PA involves the recruitment of Cbl by β-arrestin.
A: Hela cells were transfected 72 hours with control siRNAs or siRNAs against Cbl, AP-1, AP-2, β-arrestin-1 (β-Arr-1), or β-arrestin-2 (β-Arr-2). Cells were treated with 1 µg/ml of PA63 for 1 hr at 4°C (red) and 30 min at 37°C (blue) and subsequently submitted to FACS analysis. B: Hela cells were transfected 72 hrs with TEM8/1-HA and with control siRNAs or siRNAs against β-arrestin 2. Cells were then treated or not with 1 µg/ml of PA63 WT for 1 hr at 4°C and different times at 37°C. Immunoprecipitates against TEM8-HA were analyzed by SDS-PAGE and western blotting against Ubiquitin, TEM8-HA and PA. C: Hela cells were transfected or not for 24 hrs with TEM8/1-HA. Immunoprecipitates against β-arrestin were analyzed by SDS-PAGE and western blotting against Cbl, TEM8-HA and β-arrestin. D: Hela cells were transfected 72 hours with human CMG2-HA and with control siRNAs or siRNAs against β-arrestin 2. Cells were then treated or not with 1 µg/ml of PA63 WT for 1 hr at 4°C and different times at 37°C. Immunoprecipitates against CMG2-HA were analyzed by SDS-PAGE and western blotting against Ubiquitin, CMG2-HA and PA.
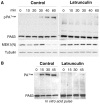
Figure 4. Latrunculin A inhibits anthrax toxin uptake.
Hela cells were treated 45 min at 37°C with or without Latrunculin A prior to addition of 1 µg/ml of PA63 for 1 hr at 4°C (red) followed by different incubation times at 37°C. A: Cells were then submitted to FACS analysis. B: The plot represents the mean of the percentage of PA at the cell surface for 4 experiments. Errors represent standard deviations.
Figure 5. Latrunculin A prevents transport of PA to endosomes and subsequent cleavage of MEK1.
A: Hela cells were treated 45 min at 37°C with or without Latrunculin A, prior to the addition of 500 ng/ml of PA63 and 100 ng/ml LF for 1 hr at 4°C followed by different incubation times at 37°C. Cell extracts (40 µg of proteins) were analyzed by SDS-PAGE and western blotting to reveal pPA7mer, PA63 and the N-terminus of MEK1 (MEK1 (N)). Tubulin is the equal loading control. B: Cell extracts (40 µg of proteins) described in A were treated 10 min at room temperature with acid buffer pH 4.5 and analyzed by SDS-PAGE and western blotting to reveal the total heptameric PA63 (PA7mer) population and PA63 monomer.
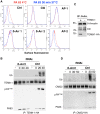
Figure 6. Binding of PA leads to the release of the actin-interacting complex form the cytosolic tail of TEM8.
Hela cells were transfected 24 hrs with TEM8/1-HA (ABC) or TEM8/2-HA (B) or CMG2 (B) or constructs CTT (B) or CCT (B) or TEM8/1 Y383C-HA (C). Cells were then treated or not with 1 µg/ml of PA83 WT (ABC) or mutant resistant to furin cleavage (C) for 1 hr at 4°C. A: Cells were subsequently incubated 5 or 20 minutes at 37°C. Immunoprecipitates against TEM8-HA were analyzed by SDS-PAGE and western blotting against Actin, Talin, Vinculin, TEM8-HA and PA. B: Cells were subsequently incubated 10 minutes at 37°C. Immunoprecipitates against HA were analyzed by SDS-PAGE and western blotting against Vinculin, Actin and HA. C: Cells were subsequently incubated 10 minutes at 37°C. Immunoprecipitates against TEM8-HA were analyzed by SDS-PAGE and western blotting against Actin, TEM8-HA and PA.
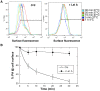
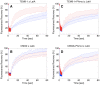
Figure 7. Effect of latrunculin A on the surface mobility of TEM8-1 and CMG2.
Hela cells were transfected 48 hrs with TEM8-1-GFP (AC) or CMG2-GFP (BD) and treated (red curves) or not (blue curves) with Latrunculin A. AB: Cells were submitted to FRAP analysis in the absence of toxin treatment. CD: Cells were incubated with 3 µg/ml of mutant PA, resistant to furin cleavage and thus defective in heptamerization, at 4°C for 1 hr and subsequently warmed back to room temperature, in the presence or absence of latrunculin A, for FRAP analysis. Each curve is the average of at least 9 different cells. Error bars represent standard deviations of the mean at each time point.
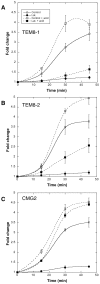
Figure 8. Receptor dependent actin requirements for PA heptamerization and endocytosis.
CHOΔATR cells were transfected 48 hrs with TEM8/1-HA (A) or TEM8/2-HA (B) or CMG2/4-V5 (C) or empty pCDNA3 plasmid (control). Cells were then treated 45 min at 37°C with (filled symbols) or without (open symbols) Latrunculin A, prior to the addition of 500 ng/ml of PA63 and 100 ng/ml LF for 1 hr at 4°C followed by different incubation times at 37°C. Total cell extracts were either analyzed directly by SDS-PAGE and western blotting against PA or first treated 10 min at room temperature with acid buffer to reveal the total PA63 heptameric population (surface + intracellular: dashed lines). PA7mer and pPA7mer levels were quantified using the Typhoon scanner and normalized to 1 at the level at time 0 (1 hour at 4°C). The plot represents the mean of 3 independent experiments. Errors represent standard deviations.
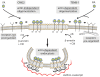
Figure 9. Schematic representation of the endocytosis of the anthrax toxin.
TEM8-1 is pre-organized at the cell surface by the cortical actin cytoskeleton while CMG2 is not. Upon PA binding, processing and oligomerization, the toxin receptor complex moves to lipid rafts. There, β-arrestin mediates the recruitment of the E3 ligase Cbl to the cytoplasmic tail of the receptor. The ubiquinated receptor subsequently recruits the heterotetrameric adaptor AP-1 and finally clathrin. Completion of the endocytic process and pinching off of the toxin containing clathrin-coated vesicle requires both actin and dynamin.
Similar articles
- Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process.
Abrami L, Liu S, Cosson P, Leppla SH, van der Goot FG. Abrami L, et al. J Cell Biol. 2003 Feb 3;160(3):321-8. doi: 10.1083/jcb.200211018. Epub 2003 Jan 27. J Cell Biol. 2003. PMID: 12551953 Free PMC article. - Selection of anthrax toxin protective antigen variants that discriminate between the cellular receptors TEM8 and CMG2 and achieve targeting of tumor cells.
Chen KH, Liu S, Bankston LA, Liddington RC, Leppla SH. Chen KH, et al. J Biol Chem. 2007 Mar 30;282(13):9834-9845. doi: 10.1074/jbc.M611142200. Epub 2007 Jan 24. J Biol Chem. 2007. PMID: 17251181 Free PMC article. - Effects of dynamin inactivation on pathways of anthrax toxin uptake.
Boll W, Ehrlich M, Collier RJ, Kirchhausen T. Boll W, et al. Eur J Cell Biol. 2004 Jul;83(6):281-8. doi: 10.1078/0171-9335-00373. Eur J Cell Biol. 2004. PMID: 15511085 - Anthrax toxin rafts into cells.
Kurzchalia T. Kurzchalia T. J Cell Biol. 2003 Feb 3;160(3):295-6. doi: 10.1083/jcb.200301032. J Cell Biol. 2003. PMID: 12566425 Free PMC article. Review. - The Ins and Outs of Anthrax Toxin.
Friebe S, van der Goot FG, Bürgi J. Friebe S, et al. Toxins (Basel). 2016 Mar 10;8(3):69. doi: 10.3390/toxins8030069. Toxins (Basel). 2016. PMID: 26978402 Free PMC article. Review.
Cited by
- Refining S-acylation: Structure, regulation, dynamics, and therapeutic implications.
Anwar MU, van der Goot FG. Anwar MU, et al. J Cell Biol. 2023 Nov 6;222(11):e202307103. doi: 10.1083/jcb.202307103. Epub 2023 Sep 27. J Cell Biol. 2023. PMID: 37756661 Free PMC article. - When cells and microbes meet in Krakow.
Rajalingam K, van der Goot FG. Rajalingam K, et al. EMBO Rep. 2011 Mar;12(3):188-90. doi: 10.1038/embor.2011.27. Epub 2011 Feb 25. EMBO Rep. 2011. PMID: 21350503 Free PMC article. - Endocytic trafficking of membrane-bound cargo: a flotillin point of view.
Meister M, Tikkanen R. Meister M, et al. Membranes (Basel). 2014 Jul 11;4(3):356-71. doi: 10.3390/membranes4030356. Membranes (Basel). 2014. PMID: 25019426 Free PMC article. - Binding of filamentous actin to anthrax toxin receptor 1 decreases its association with protective antigen.
Garlick KM, Batty S, Mogridge J. Garlick KM, et al. Biochemistry. 2012 Feb 14;51(6):1249-56. doi: 10.1021/bi2016469. Epub 2012 Feb 3. Biochemistry. 2012. PMID: 22303962 Free PMC article. - Hemoglobin S and C affect protein export in Plasmodium falciparum-infected erythrocytes.
Kilian N, Srismith S, Dittmer M, Ouermi D, Bisseye C, Simpore J, Cyrklaff M, Sanchez CP, Lanzer M. Kilian N, et al. Biol Open. 2015 Feb 20;4(3):400-10. doi: 10.1242/bio.201410942. Biol Open. 2015. PMID: 25701664 Free PMC article.
References
- Abrami L, Reig N, van der Goot FG. Anthrax toxin: the long and winding road that leads to the kill. Trends Microbiol. 2005;13:72–78. - PubMed
- Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. - PubMed
- Scobie HM, Young JA. Interactions between anthrax toxin receptors and protective antigen. Curr Opin Microbiol. 2005;8:106–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous