Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-kappaB - PubMed (original) (raw)
Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-kappaB
H Boukerche et al. Oncogene. 2010.
Abstract
The scaffolding postsynaptic density-95/disks large/zonula occludens-1 (PDZ) domain-containing protein melanoma differentiation associated gene-9 (MDA-9)/syntenin is a tandem PDZ protein overexpressed in human melanoma, and breast and gastric cancer cells. MDA-9/syntenin affects cancer cell motility and invasion through distinct biochemical and signaling pathways, including focal adhesion kinase and p38 mitogen-activated protein kinase (MAPK), resulting in activation of the nuclear factor (NF)-kappaB pathway. MDA-9/syntenin also promotes melanoma metastasis by activating c-Src, but how c-Src regulates NF-kappaB activation is unclear. Using a human melanoma model, we document that MDA-9/syntenin-c-Src interactions are positive regulators of NF-kappaB activation. Inhibition of c-Src by PP2 treatment, by blocking c-Src or mda-9/syntenin expression with small interfering RNA, or in c-Src (-/-) knockout cell lines, reduces NF-kappaB activation following overexpression of mda-9/syntenin or c-Src. Deletion or point mutations of the PDZ binding motif preventing MDA-9/syntenin association with c-Src reveals that both PDZ domains, with PDZ2 being the dominant module, are required for activating downstream signaling pathways, including p38 MAPK and NF-kappaB. We also document that MDA-9/syntenin-c-Src complexes functionally cooperate with NF-kappaB to promote anchorage-independent growth, motility and invasion of melanoma cells. These findings underscore PDZ domains of MDA-9/syntenin as promising potential therapeutic targets for intervening in a decisive component of cancer progression, namely, metastatic tumor spread.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures
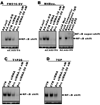
Figure 1. Pharmacological inhibition of c-Src reduces _mda-_9/syntenin-mediated activation of the NF-κB signaling pathway in immortal human melanocytes and human melanoma cell lines derived from tumors at different stages of tumor progression
(A,B) IκBα degradation in melanoma cells is decreased by PP2 treatment, but by PP3 treatment. FM516-SV or M4Beu. cells (weakly metastatic melanoma cells) were infected with either Ad.null (adenovirus vector lacking a gene insert) or Ad._mda-_9/S at a MOI of 50 pfu/cell. Twelve h later, cells were transfected with c-Src siRNA or an irrelevant siRNA control RNA duplex. After 48 h, cells were plated on fibronectin in serum-free medium in the absence or presence of increasing concentratons of the active pharmacological Src inhibitor PP2 or the inactice analog PP3. Whole cell lysates were analyzed by immunoblotting with anti-IκBα and anti-EF1α antibodies. (C and D) _mda-_9/syntenin-induced NF-κB promoter activity decreases following PP2 treatment of melanoma cells. FM516-SV, M4Beu., or T1P26 and 7GP (highly metastatic melanoma cells) cells were either uninfected or infected with Ad.null, Ad._mda-_9/S or Ad._mda-_9/AS at 50 pfu/cell. Twelve h later, cells were transfected with the indicated reporter constructs and then seeded on fibronectin-coated plates in serum-free medium in the presence or absence of the active Src inhibitor PP2, or its inactive analog PP3. Luciferase activity was measured as described in “Experimental Procedures.” Columns, mean; bars, S.D. No reporter induction was seen in Ad._null_-, or Ad._mda_-9/S-infected cells that were transfected with either pGL3Basic or the 3kBmut-Luc constructs in the absence or the presence of PP2 or PP3.
Figure 2. c-Src siRNAs regulate NF-κB binding activity in human melanoma cells
(A and B) _mda-_9/syntenin-induced NF-κB promoter activity decreases following c-Src siRNA treatment of melanoma cells. FM516-SV or M4Beu. cells were either uninfected or infected with Ad.null or Ad._mda-_9/S at 50 pfu/cell. Twelve h later, cells were transfected with Src siRNAs (10 or 50 nM) or control non specific siRNAs. Adding a 100-fold excess of unlabeled consensus NF-κB oligomer cold competitor (cold comp) completely inhibited binding, and a 100-fold excess of unlabeled unrelated sequence (cold mutant; cold mut) had no effect. Arrows refer to supershifted bands. Nuclear extracts from infected M4Beu. cells (100 pfu/cell) plated on fibronectin in serum-free medium were incubated with double-stranded 32P-labeled consensus NF-κB oligonucleotides followed by incubation with polyclonal antibody against the p65 or p50 NF-κB subunits or with control rabbit IgG. (C and D) T1P26 and 7GP cells were transfected with the indicated c-Src siRNAs. After 48 h, cells were plated on fibronectin in serum-free medium for 2 to 4 h and nuclear NF-κB binding activity was then assessed by EMSA as described in “Experimental Procedures.” UTR, untreated control.
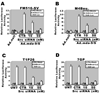
Figure 3. c-Src siRNAs regulate NF-κB transcriptional activity in human melanoma cells
(A and B) c-Src siRNA inhibits _mda-_9/syntenin-induced transcriptional activation of NF-κB. FM516-SV or M4Beu. cells were either infected with Ad.null or Ad._mda-_9/S at 50 pfu/cell. Twelve h later, cells were cotransfected with either c-Src siRNAs (10 or 50 nM) or control non-specific siRNAs along with a NF-κB-responsive luciferase reporter construct. (C and D) T1P26 and 7GP cells were transfected with the indicated c-Src siRNAs. After 48 h, cells were seeded on fibronectin-coated plates in serum-free medium. Luciferase activity was measured as described in “Materials and methods.” Columns, mean; bars, S.D.
Figure 4. Genetic ablation of SFKs or inhibition of expression of _mda-_9/syntenin in Src knockout mouse fibroblasts (SYF) abrogates _mda_-9/syntenin-mediated activation of NF-κB
(A) Src −/− Yes −/− Fyn −/− mouse embryo fibroblast cells (SYF), mouse embryo fibroblast cells expressing endogeneous Src (Src+/+), SYF cells transfected with c-Src (WT c-Src), and SYF cotransfected with c-Src and mda-9/siRNAs (50 nM) or control non-specific siRNAs were either uninfected or infected with Ad.null or Ad._mda-_9/AS at 50 pfu/cell. After 48 h, cells were seeded on fibronectin-coated plates in serum-free medium. Luciferase activity was measured as described in “Materials and methods.” Columns, mean; bars, S.D.
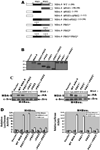
Figure 5. Mapping of c-Src binding sites involved in MDA-9/syntenin-induced NF-κB activation in human melanoma cells
(A) Schematic of deletion and mutant constructs of MDA-9/syntenin. Numbers, amino acid positions. Black boxes, PDZ1 domain. Gray boxes, PDZ2 domain. All mutants were engineered with an N-terminal HA-epitope tag. (B) Confirmation of authenticity of the generated constructs. M4Beu. cells were transfected with the indicated _mda_-9/syntenin deletion or mutant constructs and the expression of the protein products were analyzed in the cell lysates by Western blotting using anti-HA antibody. (C) Both PDZ domains of MDA-9/syntenin contribute to c-Src binding. M4Beu. cells were cotransfectd with the indicated HA-tagged MDA-9/syntenin deletion or mutant constructs. After 48 h, cells were seeded on fibronectin-coated plates in serum-free medium, and immunoprecipitated with anti-c-Src antibodies followed by Western blotting with HA-MDA-9/syntenin antibodies or anti-c-Src antibodies. (D) Both PDZ domains of MDA-9/syntenin are required for NF-κB activation. M4Beu. cells were cotransfected with the MDA-9/syntenin deletion or mutant construct and with a NF-κB-responsive luciferase reporter construct. After 48 h, cells were seeded on fibronectin-coated plates in serum-free medium. Luciferase activity was measured as described in “Materials and methods.” Columns, mean; bars, S.D.
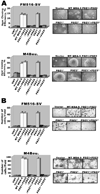
Figure 6. Cell migration/invasion and anchorage-independent growth of melanoma cells depends on both PDZ domains of MDA-9/syntenin that interact with c-Src and activate NF-κB
(A) Both PDZ domains of MDA-9/syntenin are required for migration/invasion of melanoma cells. FM516-SV or M4Beu. cells were transfected with the indicated MDA-9/syntenin deletion or mutant construct. After 48 h, cells were analyzed for migration/invasion by Matrigel invasion assay using a modified Boyden’s chamber as described in “Materials and methods.” Ten fields per cell line were counted. Columns, mean of triplicate samples from three independent experiments; bars, S.D. (left). Representative photomicrographs of cell migration/invasion taken 48 h after seeding in Matrigel (right). (B) Anchorage independent growth of FM516-SV or M4Beu. cells either untransfected or transfected with an empty vector or the indicated MDA-9/syntenin deletion or mutant construct. Forty-eight h later, 1 × 105 cells were replated in complete medium containing 0.6% agar on top of a 0.6% agar base layer. Macroscopic colonies were scored after two weeks. Columns, mean of triplicate samples from three independent experiments; bars S.D. (left). Representative photomicrographs of colonies two weeks after seeding in agar (right).
Figure 7. Both PDZ domains of MDA-9/syntenin are required for _mda-_9/syntenin-mediated activation of MMP-2 and p38 MAPK signaling
(A) M4Beu. cells were either untransfected or transfected with an empty vector or the indicated MDA-9/syntenin deletion or mutant construct. Cells were either uninfected or infected with Ad.null, Ad._mda-_9/S or Ad._mda-_9/S + Ad.IκBα-mt32 (50 pfu/cell of each virus). Forty-eight h later, total RNA was extracted and RT-PCR was performed with primer pairs to amplify MMP-2 or glyceraldehyde-3-phosphate-dehydrogenase (GAPDH). (B) M4Beu. cells were either transfected or infected with the indicated MDA-9/syntenin mutant or adenovirus construct. Forty-eight h later, cells were replated on fibronectin in serum-free medium. The conditioned medium was collected and processed by gelatin zymography as described in “Materials and methods.” (C) Serum-starved M4Beu. cells either untransfected or transfected with the indicated MDA-9/syntenin deletion or mutant construct were plated on fibronectin. Cell lysates were analyzed by Western blotting and stained with phosphospecific antibodies to p38 MAPK. Membranes were reprobed with specific antibodies directed against total enzyme. (D) RT-PCR was performed with primer pairs to amplify MMP-2 or GAPDH in M4Beu. cells transfected with the indicated MDA-9/syntenin mutant constructs. (E) Serum-starved T1P26 cells either treated with dimethyl sulfoxide vehicle (DMSO), or 5 µmol/L of SB203580, or infected with Ad.null or an adenovirus expressing a dominant-negative p38 MAPK mutant (Ad.p38α.DN) and plated on fibronectin. Cell lysates were analyzed by Western blotting and stained with anti-c-Src Tyr416, or anti-c-Src antibodies. (F) T1P26 cells either uninfected or infected with Ad.null or Ad.p38α.DN were transfected twelve h later with a NF-κB-responsive luciferase reporter construct. Forty-eight h later, cells were replated on fibronectin in serum-free medium in the presence of dimethyl sulfoxide (DMSO) vehicle control, or 5 µmol/L of SB203580. Luciferase activity was measured as described in “Materials and methods.” Columns, mean; bars, ± S.D. (G) T1P26 cells transfected with either c-Src siRNAs (2, 10 or 50 nM) or control non-specific siRNAs were seeded on fibronectin-coated plates in serum-free medium. Cell lysates were analyzed by Western blotting and stained with phosphospecific antibodies to p38 MAPK. Membranes were reprobed with specific antibodies directed against total enzyme.
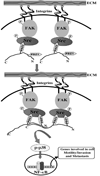
Fig. 8. Hypothetical model of MDA-9/syntenin-mediated induction of NF-κB and its downstream genes and processes through its interaction with c-Src
Upon interacting with the ECM (fibronectin), MDA-9/syntenin physically interacts with c-Src in a highly cooperative manner, with the PDZ2 being the dominant motif then interacting with PDZ1 resulting in the assembly of MDA-9/syntenin into a multimeric complexes and consequently a more stable functional unit. These interactions also involve binding of the NH2 terminus of MDA-9/syntenin within the COOH-terminal two thirds of the molecule and enables "head to tail" association (Koroll et al., 2001). MDA-9/syntenin interactions with c-Src assemble c-Src/FAK signaling complexes at high density at the plasma membrane and leads to activation of the p38 MAPK/NF-κB pathway that regulates expression of genes involved in cell motility and invasion and thus plays a decisive role in MDA-9/syntenin-mediated tumor progression and metastasis.
Similar articles
- RETRACTED: mda-9/Syntenin regulates the metastatic phenotype in human melanoma cells by activating nuclear factor-kappaB.
Boukerche H, Su ZZ, Emdad L, Sarkar D, Fisher PB. Boukerche H, et al. Cancer Res. 2007 Feb 15;67(4):1812-22. doi: 10.1158/0008-5472.CAN-06-3875. Cancer Res. 2007. PMID: 17308124 Retracted. - MDA-9/syntenin is essential for factor VIIa-induced signaling, migration, and metastasis in melanoma cells.
Aissaoui H, Prévost C, Boucharaba A, Sanhadji K, Bordet JC, Négrier C, Boukerche H. Aissaoui H, et al. J Biol Chem. 2015 Feb 6;290(6):3333-48. doi: 10.1074/jbc.M114.606913. Epub 2014 Dec 10. J Biol Chem. 2015. PMID: 25505176 Free PMC article. Retracted. - mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src.
Boukerche H, Su ZZ, Prévot C, Sarkar D, Fisher PB. Boukerche H, et al. Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):15914-9. doi: 10.1073/pnas.0808171105. Epub 2008 Oct 2. Proc Natl Acad Sci U S A. 2008. PMID: 18832467 Free PMC article. - Syntenin: a novel PDZ domain-containing scaffolding protein associated with human melanoma metastasis.
Yang JB, McCarthy JB. Yang JB, et al. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007 Apr;32(2):204-12. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007. PMID: 17478924 Review. - Targeting tumor invasion: the roles of MDA-9/Syntenin.
Kegelman TP, Das SK, Emdad L, Hu B, Menezes ME, Bhoopathi P, Wang XY, Pellecchia M, Sarkar D, Fisher PB. Kegelman TP, et al. Expert Opin Ther Targets. 2015 Jan;19(1):97-112. doi: 10.1517/14728222.2014.959495. Epub 2014 Sep 15. Expert Opin Ther Targets. 2015. PMID: 25219541 Free PMC article. Review.
Cited by
- Raf kinase inhibitor RKIP inhibits MDA-9/syntenin-mediated metastasis in melanoma.
Das SK, Bhutia SK, Sokhi UK, Azab B, Su ZZ, Boukerche H, Anwar T, Moen EL, Chatterjee D, Pellecchia M, Sarkar D, Fisher PB. Das SK, et al. Cancer Res. 2012 Dec 1;72(23):6217-26. doi: 10.1158/0008-5472.CAN-12-0402. Epub 2012 Oct 11. Cancer Res. 2012. PMID: 23066033 Free PMC article. - Expression patterns of MDA-9/syntenin during development of the mouse embryo.
Jeon HY, Das SK, Dasgupta S, Emdad L, Sarkar D, Kim SH, Lee SG, Fisher PB. Jeon HY, et al. J Mol Histol. 2013 Apr;44(2):159-66. doi: 10.1007/s10735-012-9468-1. Epub 2012 Nov 20. J Mol Histol. 2013. PMID: 23180153 Free PMC article. - Mda-9/syntenin is expressed in uveal melanoma and correlates with metastatic progression.
Gangemi R, Mirisola V, Barisione G, Fabbi M, Brizzolara A, Lanza F, Mosci C, Salvi S, Gualco M, Truini M, Angelini G, Boccardo S, Cilli M, Airoldi I, Queirolo P, Jager MJ, Daga A, Pfeffer U, Ferrini S. Gangemi R, et al. PLoS One. 2012;7(1):e29989. doi: 10.1371/journal.pone.0029989. Epub 2012 Jan 13. PLoS One. 2012. PMID: 22267972 Free PMC article. - Novel role of MDA-9/syntenin in regulating urothelial cell proliferation by modulating EGFR signaling.
Dasgupta S, Menezes ME, Das SK, Emdad L, Janjic A, Bhatia S, Mukhopadhyay ND, Shao C, Sarkar D, Fisher PB. Dasgupta S, et al. Clin Cancer Res. 2013 Sep 1;19(17):4621-33. doi: 10.1158/1078-0432.CCR-13-0585. Epub 2013 Jul 19. Clin Cancer Res. 2013. PMID: 23873690 Free PMC article. - The Multifunctional Protein Syntenin-1: Regulator of Exosome Biogenesis, Cellular Function, and Tumor Progression.
Lee KM, Seo EC, Lee JH, Kim HJ, Hwangbo C. Lee KM, et al. Int J Mol Sci. 2023 May 29;24(11):9418. doi: 10.3390/ijms24119418. Int J Mol Sci. 2023. PMID: 37298370 Free PMC article. Review.
References
- Baril P, Nejjari M, Scoazek JY, Boukerche H. Blocking a novel 55 kDa melanoma-associated cell surface antigen inhibits the development of spontaneous metastases and interactions with frozen lung section. Int J Cancer. 2002;99:315–322. - PubMed
- Boukerche H, Baril P, Tabone E, Bérard F, Sanhadji K, Balme B, et al. A new Mr 55,000 surface protein implicated in melanoma progression: association with a metastatic phenotype. Cancer Res. 2000;60:5848–5856. - PubMed
- Boukerche H, Su ZZ, Kang DC, Fisher PB. Identification and cloning of genes displaying elevated expression as a consequence of metastatic progression in human melanoma cells by rapid subtraction hybridization. Gene. 2004;343:191–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA259599/CA/NCI NIH HHS/United States
- R01 CA035675/CA/NCI NIH HHS/United States
- R01 CA097318-05/CA/NCI NIH HHS/United States
- R01 CA097318-06A1/CA/NCI NIH HHS/United States
- R01 CA035675-22/CA/NCI NIH HHS/United States
- CA097318/CA/NCI NIH HHS/United States
- R01 CA035675-21/CA/NCI NIH HHS/United States
- R01 CA097318/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous