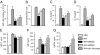Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma - PubMed (original) (raw)
Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma
Krisztian Nemeth et al. Proc Natl Acad Sci U S A. 2010.
Erratum in
- Proc Natl Acad Sci U S A. 2010 Apr 27;107(17):8041. Gorham, Jared D [corrected to Gorham, James D]; Bundoc, Victor G [corrected to Bundoc, Virgilio G]
Abstract
Bone marrow stromal cells [BMSCs; also known as mesenchymal stem cells (MSCs)] effectively suppress inflammatory responses in acute graft-versus-host disease in humans and in a number of disease models in mice. Many of the studies concluded that BMSC-driven immunomodulation is mediated by the suppression of proinflammatory Th1 responses while rebalancing the Th1/Th2 ratio toward Th2. In this study, using a ragweed induced mouse asthma model, we studied if BMSCs could be beneficial in an allergic, Th2-dominant environment. When BMSCs were injected i.v. at the time of the antigen challenge, they protected the animals from the majority of asthma-specific pathological changes, including inhibition of eosinophil infiltration and excess mucus production in the lung, decreased levels of Th2 cytokines (IL-4, IL-5, and IL-13) in bronchial lavage, and lowered serum levels of Th2 immunoglobulins (IgG1 and IgE). To explore the mechanism of the effect we used BMSCs isolated from a variety of knockout mice, performed in vivo blocking of cytokines and studied the effect of asthmatic serum and bronchoalveolar lavage from ragweed challenged animals on the BMSCs in vitro. Our results suggest that IL-4 and/or IL-13 activate the STAT6 pathway in the BMSCs resulting in an increase of their TGF-beta production, which seems to mediate the beneficial effect, either alone, or together with regulatory T cells, some of which might be recruited by the BMSCs. These data suggest that, in addition to focusing on graft-versus-host disease and autoimmune diseases, allergic conditions--specifically therapy resistant asthma--might also be a likely target of the recently discovered cellular therapy approach using BMSCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Timeline of the experiments showing the days of the interventions.
Fig. 2.
Histological images of airways stained with PAS to show the mucin-producing goblet cells (dark red in the lumen). Low-magnification images depict a control lung (A), a lung following RW challenge with no treatment (B), and a lung with BMSC treatment (C). Note the significant increase in lymphocytic infiltrates (arrows) in B and their decrease in C. The high magnification images of the airways show a normal bronchus in D, a bronchus from RW-challenged mouse with mucus buildup (arrows) in the luminal surface (E), and a treated mouse with less mucus in F. (Scale bar, 250 μm in A_–_C and 50 μm in D_–_F.)
Fig. 3.
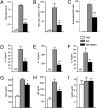
Evaluation of the effect of BMSC treatment on the different parameters of RW-induced asthma. Mice treated with BMSCs showed a significant reduction in lung histology scores (A), total number of BAL cells (B), relative ratio of BAL eosinophils (C), and levels of allergy-specific Th2 cytokines IL-4 (D), IL-13 (E), and IL5 (F) in BAL. From the sera of challenged mice we measured Ig concentrations, and in the BMSC-treated group we found a significant decrease in the level of Th2-specific Ig concentrations IgG1 (G) and IgE (H), whereas there was no change in the level of IgG2a (I). There were four to eight mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001 in all graphs.
Fig. 4.
Assessing the effect of allogeneic BMSCs or syngeneic skin fibroblasts on RW-induced asthma. Allogeneic BMSCs exhibit a similar inhibitory effect as syngeneic BMSCs on the total number of BAL cells (A), relative ratio of eosinophils (B), BAL inflammatory cytokines IL-4 (C) and IL-13 (D), and serum immunoglobulins IgG1 (E), IgE (F), and IgG2a (G). There were four to eight mice per group. Skin fibroblasts had a partial effect on the aforementioned parameters (see same graphs).
Fig. 5.
After i.v. delivery of BMSCs at the time of the first challenge, stromal cells are concentrated in the lung. Asthmatic lungs seem to retain more BMSCs than control unchallenged lungs at 48 h after administration, demonstrated by bioluminescence detection of luciferase-expressing stromal cells. Two representative mice of three used are shown (A). To confirm this observation, Q-dot–labeled BMSCs were injected at the time of the first challenge, and lung cell suspensions were analyzed using FACS at several time points after injection. After 6 h, the number of Q-dot–positive cells (BMSCs) are still comparable in the two groups (each had four mice), but starting at 12 h there are significantly more BMSCs retained in the asthmatic lungs compared with the controls at all time points examined (B and C).
Fig. 6.
Studying the mechanism of BMSC effect. In BAL BMSC treatment resulted in elevated TGF-β levels (A). IFN-γ (B) and IL-10 (C) did not change. Serum or BAL from RW challenged mice induced BMSCs to producemore TGF-β in vitro, but this effect was eliminated when BMSCs lacked IL-4Ra (D). Using neutralizing antibodies for IL-4 or IL-13 suggested both cytokines are involved in stimulating BMSC's TGF-β production when cocultured with serum (E) or BAL (F) from RW challenged mice.
Fig. 7.
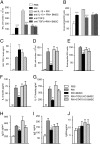
In vivo demonstration of mechanism of action. The beneficial effect of BMSCs on inflammatory changes is eliminated in the presence of TGF-β neutralizing antibodies, but spared when animals are treated with anti-IL-10 antibodies (A and B). BMSCs from TGF-β or STAT6 deficient animals did not induce TGF-β production (C) or decrease BAL total cell numbers (D), eosinophil numbers (E), cytokine levels (F and G), or serum immunoglobulin concentrations (H_–_J).
Fig. 8.
Quantification of regulatory T cells in asthmatic lungs. With time there is a gradual increase in the number of regulatory T cells in lungs challenged with RW. BMSC-treated asthmatic lungs show a greater influx of T-regs starting at 36 h after challenge and further increasing up to 96 h.
Fig. 9.
Schematic drawing shows the mechanism of effect based on data of the present study. BMSCs “sense” the allergic environment, and as a result of the increased levels of IL-4/IL-13, they respond by producing higher amounts of TGF-β that, either alone or by recruiting regulatory T cells, will ultimately lead to a decrease of lung eosinophil infiltration, as well as allergy-specific cytokine and Ig production.
Similar articles
- Simvastatin and bone marrow-derived mesenchymal stem cells (BMSCs) affects serum IgE and lung cytokines levels in sensitized mice.
Mohammadian M, Sadeghipour HR, Jahromi GP, Jafari M, Nejad AK, Khamse S, Boskabady MH. Mohammadian M, et al. Cytokine. 2019 Jan;113:83-88. doi: 10.1016/j.cyto.2018.06.016. Epub 2018 Jun 15. Cytokine. 2019. PMID: 29914792 - Immunomodulation of airway epithelium cell activation by mesenchymal stromal cells ameliorates house dust mite-induced airway inflammation in mice.
Duong KM, Arikkatt J, Ullah MA, Lynch JP, Zhang V, Atkinson K, Sly PD, Phipps S. Duong KM, et al. Am J Respir Cell Mol Biol. 2015 Nov;53(5):615-24. doi: 10.1165/rcmb.2014-0431OC. Am J Respir Cell Mol Biol. 2015. PMID: 25789608 - Indoleamine 2,3-Dioxygenase Is Not a Pivotal Regulator Responsible for Suppressing Allergic Airway Inflammation through Adipose-Derived Stem Cells.
Cho KS, Park MK, Mun SJ, Park HY, Yu HS, Roh HJ. Cho KS, et al. PLoS One. 2016 Nov 3;11(11):e0165661. doi: 10.1371/journal.pone.0165661. eCollection 2016. PLoS One. 2016. PMID: 27812173 Free PMC article. - Effect of mesenchymal stromal (stem) cell (MSC) transplantation in asthmatic animal models: A systematic review and meta-analysis.
Zhang LB, He M. Zhang LB, et al. Pulm Pharmacol Ther. 2019 Feb;54:39-52. doi: 10.1016/j.pupt.2018.11.007. Epub 2018 Nov 26. Pulm Pharmacol Ther. 2019. PMID: 30496803 - Stem cells in animal asthma models: a systematic review.
Srour N, Thébaud B. Srour N, et al. Cytotherapy. 2014 Dec;16(12):1629-42. doi: 10.1016/j.jcyt.2014.08.008. Epub 2014 Oct 18. Cytotherapy. 2014. PMID: 25442788
Cited by
- Intratracheal Administration of Mesenchymal Stem Cells Modulates Tachykinin System, Suppresses Airway Remodeling and Reduces Airway Hyperresponsiveness in an Animal Model.
Urbanek K, De Angelis A, Spaziano G, Piegari E, Matteis M, Cappetta D, Esposito G, Russo R, Tartaglione G, De Palma R, Rossi F, D'Agostino B. Urbanek K, et al. PLoS One. 2016 Jul 19;11(7):e0158746. doi: 10.1371/journal.pone.0158746. eCollection 2016. PLoS One. 2016. PMID: 27434719 Free PMC article. - Effect of TGF-β1 Stimulation on the Secretome of Human Adipose-Derived Mesenchymal Stromal Cells.
Rodríguez TM, Saldías A, Irigo M, Zamora JV, Perone MJ, Dewey RA. Rodríguez TM, et al. Stem Cells Transl Med. 2015 Aug;4(8):894-8. doi: 10.5966/sctm.2015-0012. Epub 2015 May 29. Stem Cells Transl Med. 2015. PMID: 26025982 Free PMC article. - Pollen/TLR4 Innate Immunity Signaling Initiates IL-33/ST2/Th2 Pathways in Allergic Inflammation.
Li J, Zhang L, Chen X, Chen D, Hua X, Bian F, Deng R, Lu F, Li Z, Pflugfelder SC, Li DQ. Li J, et al. Sci Rep. 2016 Oct 31;6:36150. doi: 10.1038/srep36150. Sci Rep. 2016. PMID: 27796360 Free PMC article. - Embryonic-stem-cell-derived mesenchymal stem cells relieve experimental contact urticaria by regulating the functions of mast cells and T cells.
Hyun SY, Kim EY, Kang M, Park JW, Hong KS, Chung HM, Choi WS, Park SP, Noh G, Kim HS. Hyun SY, et al. Sci Rep. 2023 Dec 20;13(1):22694. doi: 10.1038/s41598-023-50258-2. Sci Rep. 2023. PMID: 38123643 Free PMC article. - Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery.
Porada CD, Almeida-Porada G. Porada CD, et al. Adv Drug Deliv Rev. 2010 Sep 30;62(12):1156-66. doi: 10.1016/j.addr.2010.08.010. Epub 2010 Sep 7. Adv Drug Deliv Rev. 2010. PMID: 20828588 Free PMC article. Review.
References
- Chung KF, et al. European Respiratory Society. Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma. Eur Respir J. 1999;13:1198–1208. - PubMed
- Le Blanc K, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. - PubMed
- Le Blanc K, Ringdén O. Use of mesenchymal stem cells for the prevention of immune complications of hematopoietic stem cell transplantation. Haematologica. 2005;90:438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous