Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis - PubMed (original) (raw)
Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis
Poh-Yi Gan et al. J Am Soc Nephrol. 2010 Jun.
Abstract
A major target autoantigen in anti-neutrophil cytoplasmic antibody-associated vasculitis is myeloperoxidase (MPO). Although MPO-specific CD4+ Th cells seem to orchestrate renal injury, the role of the Th17 subset is unknown. We hypothesized that Th17 cells direct injurious anti-MPO autoimmunity in experimental murine anti-MPO-induced glomerulonephritis (GN). We immunized mice with MPO to establish autoimmunity, resulting in systemic IL-17A production with MPO-specific dermal delayed-type hypersensitivity. We triggered disease using antibodies to the glomerular basement membrane to induce glomerular deposition of MPO by neutrophils. Wild-type mice developed necrotizing GN with an influx of glomerular leukocytes and albuminuria. In contrast, mice deficient in the key Th17 effector cytokine IL-17A were nearly completely protected. The protective effects resulted partly from reduced neutrophil recruitment, which led to less disposition of glomerular MPO. To test whether IL-17A also drives autoimmune delayed-type hypersensitivity in the kidney, we injected MPO into the kidneys of MPO-sensitized mice. IL-17A deficiency reduced accumulation of renal macrophages and renal CCL5 mRNA expression. In conclusion, IL-17A contributes to the pathophysiology of autoimmune anti-MPO GN, suggesting that it may be a viable therapeutic target for this disease.
Figures
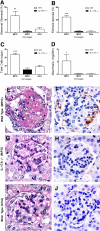
Figure 1.
Immunization with nmMPO induces comparable levels of anti-nmMPO IgG in WT (n = 6) and IL-17A−/− mice (n = 6). (A) Levels of anti-nmMPO antibody titers in OVA-immunized mice (n = 5) are similar to normal mouse serum (0.059 OD 450 nm). (B) Re-stimulation of splenocytes with MPO induces IL-17A production in MPO-immunized WT mice but not in OVA-immunized mice. As expected, no IL-17A was detected in IL-17A−/− mice. (C) No significantly different IFN-γ production could be detected between groups. (D) Antigen-specific dermal footpad DTH responses were observed in MPO-immunized WT mice and absent in IL-17A−/− mice and OVA-immunized MPO-challenged control mice. ***P < 0.001 versus MPO-immunized IL-17A−/− and OVA-immunized WT controls.
Figure 2.
Renal injury occurred in mice with autoimmune anti-MPO focal necrotizing GN. (A through D) MPO-immunized WT mice (n = 6) showed significant glomerular injury with pronounced presence of abnormal glomeruli (A), fibrin deposition (B), glomerular hypercellularity (C), and elevated albuminuria (baseline levels of albuminuria from naive WT mice being 0.13 mg/24 h; D). Protection from glomerular injury was observed in IL-17A−/− mice (n = 6), and renal injury was similar to OVA-immunized control mice (n = 5). (E through J) Illustrative examples of periodic acid-Schiff and fibrin staining between groups of mice shown, respectively; MPO-immunized WT mice (E and F), MPO-immunized IL-17A−/− mice (G and H), and OVA-immunized WT mice (I and J). *P < 0.05, **P < 0.01, ***P < 0.001 versus MPO-immunized IL-17A−/− and OVA-immunized WT controls.
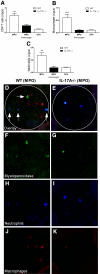
Figure 3.
Cellular effectors of injury accumulate in WT and IL-17A−/− mice. (A through C) MPO-immunized WT mice (n = 6) with autoimmune anti-MPO GN showed significantly higher numbers of renal CD4+ T cells (A), macrophages (B), and neutrophils (C) when compared with OVA-immunized mice (n = 5). Accumulation of these cells was largely IL-17A dependent. (D, F, H, and J) In WT mice, triple staining for MPO (F, green) neutrophils (H, blue), and macrophages (J, red) showed that some of the MPO molecules co-localized with neutrophils (vertical arrows, D), whereas some were associated with neither neutrophils nor macrophages (horizontal arrow, D). (G, I, and K) In the absence of IL-17A, the autoantigen, MPO was markedly diminished in glomeruli (G), as were glomerular neutrophils (I) and macrophages (K). (E) The overlay. **P < 0.01, ***P < 0.001 versus MPO-immunized IL-17A−/− and OVA-immunized WT controls.
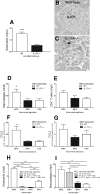
Figure 4.
IL-17A is important in localizing both neutrophils and macrophages to the kidney in experimental anti-MPO disease. After injection of anti-GBM antibodies to naive WT and IL-17A−/− mice (n = 6), neutrophils accumulated within glomeruli by 2 hours. (A through C) This neutrophil accumulation was diminished in the absence of IL-17A. ***P < 0.001 _versus_ IL-17A−/− mice. (D) Intrarenal injection of MPO to MPO-immunized mice (_n_ = 7) results in macrophage accumulation in the kidney, which is abrogated in the absence of IL-17A (_n_ = 6). *_P_ < 0.05 _versus_ MPO-immunized, MPO-injected 1L-17A−/− mice and OVA-injected WT mice. (E) Reductions in numbers of CD4+ cells are NS. (F and G) IL-17A deficiency is associated with significantly reduced renal mRNA expression of CCL5 and a trend toward lower CCL2 expression in MPO-injected kidneys relative to MPO-immunized WT mice that were administered an intrarenal injection of OVA. (H) Neutrophil depletion before intrarenal injection of MPO in MPO-immunized WT and IL-17A−/− mice (_n_ = 6) resulted in >90% depletion in kidneys. ***P < 0.001. Treatment did not alter macrophage recruitment compared with nontreated MPO-immunized, MPO intrarenally WT mice (n = 6). Macrophage recruitment was significantly reduced in kidneys of IL-17A−/− mice. *P < 0.05; **P < 0.01.
Comment in
- IL-17A in experimental glomerulonephritis: where does it come from?
Free ME, Falk RJ. Free ME, et al. J Am Soc Nephrol. 2010 Jun;21(6):885-6. doi: 10.1681/ASN.2010040408. Epub 2010 May 13. J Am Soc Nephrol. 2010. PMID: 20466743 No abstract available.
Similar articles
- Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis.
Ruth AJ, Kitching AR, Kwan RY, Odobasic D, Ooi JD, Timoshanko JR, Hickey MJ, Holdsworth SR. Ruth AJ, et al. J Am Soc Nephrol. 2006 Jul;17(7):1940-9. doi: 10.1681/ASN.2006020108. Epub 2006 Jun 12. J Am Soc Nephrol. 2006. PMID: 16769746 - IL-17A in experimental glomerulonephritis: where does it come from?
Free ME, Falk RJ. Free ME, et al. J Am Soc Nephrol. 2010 Jun;21(6):885-6. doi: 10.1681/ASN.2010040408. Epub 2010 May 13. J Am Soc Nephrol. 2010. PMID: 20466743 No abstract available. - Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis.
Odobasic D, Kitching AR, Semple TJ, Holdsworth SR. Odobasic D, et al. J Am Soc Nephrol. 2007 Mar;18(3):760-70. doi: 10.1681/ASN.2006040375. Epub 2007 Jan 31. J Am Soc Nephrol. 2007. PMID: 17267745 - Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis.
Rutgers A, Slot M, van Paassen P, van Breda Vriesman P, Heeringa P, Tervaert JW. Rutgers A, et al. Am J Kidney Dis. 2005 Aug;46(2):253-62. doi: 10.1053/j.ajkd.2005.05.003. Am J Kidney Dis. 2005. PMID: 16112043 Review. - Review: T helper 17 cells: their role in glomerulonephritis.
Ooi JD, Kitching AR, Holdsworth SR. Ooi JD, et al. Nephrology (Carlton). 2010 Aug;15(5):513-21. doi: 10.1111/j.1440-1797.2010.01343.x. Nephrology (Carlton). 2010. PMID: 20649870 Review.
Cited by
- The Th17 Pathway in Vascular Inflammation: Culprit or Consort?
Robert M, Miossec P, Hot A. Robert M, et al. Front Immunol. 2022 Apr 11;13:888763. doi: 10.3389/fimmu.2022.888763. eCollection 2022. Front Immunol. 2022. PMID: 35479069 Free PMC article. Review. - NF-κB in inflammation and renal diseases.
Zhang H, Sun SC. Zhang H, et al. Cell Biosci. 2015 Nov 16;5:63. doi: 10.1186/s13578-015-0056-4. eCollection 2015. Cell Biosci. 2015. PMID: 26579219 Free PMC article. Review. - The immunodominant myeloperoxidase T-cell epitope induces local cell-mediated injury in antimyeloperoxidase glomerulonephritis.
Ooi JD, Chang J, Hickey MJ, Borza DB, Fugger L, Holdsworth SR, Kitching AR. Ooi JD, et al. Proc Natl Acad Sci U S A. 2012 Sep 25;109(39):E2615-24. doi: 10.1073/pnas.1210147109. Epub 2012 Sep 5. Proc Natl Acad Sci U S A. 2012. PMID: 22955884 Free PMC article. - Thymic deletion and regulatory T cells prevent antimyeloperoxidase GN.
Tan DS, Gan PY, O'Sullivan KM, Hammett MV, Summers SA, Ooi JD, Lundgren BA, Boyd RL, Scott HS, Kitching AR, Chidgey AP, Holdsworth SR. Tan DS, et al. J Am Soc Nephrol. 2013 Mar;24(4):573-85. doi: 10.1681/ASN.2012090898. Epub 2013 Feb 7. J Am Soc Nephrol. 2013. PMID: 23393320 Free PMC article.
References
- Morgan MD, Harper L, Williams J, Savage C: Anti-neutrophil cytoplasm-associated glomerulonephritis. J Am Soc Nephrol 17: 1224–1234, 2006. - PubMed
- Little MA, Pusey CD: Glomerulonephritis due to antineutrophil cytoplasm antibody-associated vasculitis: An update on approaches to management. Nephrology (Carlton) 10: 368–376, 2005. - PubMed
- Ruth AJ, Kitching AR, Kwan RY, Odobasic D, Ooi JD, Timoshanko JR, Hickey MJ, Holdsworth SR: Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 17: 1940–1949, 2006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous