Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration - PubMed (original) (raw)
Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration
Honghong Yao et al. Blood. 2010.
Abstract
Cocaine abuse hastens the neurodegeneration often associated with advanced HIV-1 infection. The mechanisms, in part, revolve around the neuroinflammatory processes mediated by the chemokine monocyte chemotactic protein-1 (MCP-1/CCL2). Understanding factors that modulate MCP-1 and, in turn, facilitate monocyte extravasation in the brain is thus of paramount importance. We now demonstrate that cocaine induces MCP-1 in rodent microglia through translocation of the sigma receptor to the lipid raft microdomains of the plasma membrane. Sequential activation of Src, mitogen-activated protein kinases (MAPKs), and phosphatidylinositol-3' kinase (PI3K)/Akt and nuclear factor kappaB (NF-kappaB) pathways resulted in increased MCP-1 expression. Furthermore, conditioned media from cocaine-exposed microglia increased monocyte transmigration, and thus was blocked by antagonists for CCR2 or sigma receptor. These findings were corroborated by demonstrating increased monocyte transmigration in mice exposed to cocaine, which was attenuated by pretreatment of mice with the sigma receptor antagonist. Interestingly, cocaine-mediated transmigratory effects were not observed in CCR2 knockout mice. We conclude that cocaine-mediated induction of MCP-1 accelerates monocyte extravasation across the endothelium. Understanding the regulation of MCP-1 expression and functional changes by cocaine/sigma receptor system may provide insights into the development of potential therapeutic targets for HIV-1-associated neurocognitive disorders.
Figures
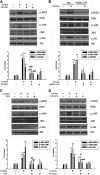
Figure 1
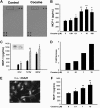
Cocaine induces MCP-1 mRNA and protein expression in microglia. (A) Supernatant fluids from cocaine-treated cells were assessed for release of cytokine/chemokines using the mouse cytokine antibody array. Cocaine treatment resulted in induction of MCP-1 expression (rectangles). (B) Concentration curve of cocaine-mediated induction of MCP-1 expression in BV-2 cells. Cells were incubated with various concentrations of cocaine (0.01, 0.1, 1, 10, and 100μM) for 12 hours, followed by collection of media for assay of MCP-1 expression by ELISA. (C) Time dependence of cocaine-mediated induction of MCP-1 expression in BV-2 cells. (D) Cocaine-mediated induction of MCP-1 mRNA expression by real time RT-PCR. (E) Representative picture of Iba-1 (microglia marker) staining in rat primary microglia. (F) Concentration curve of cocaine-mediated (0.1, 1, and 10μM) induction of MCP-1 expression in rat primary cultured microglia. All the data are presented as means ± SD of 4 individual experiments. *P < .05, **P < .01 versus control group.
Figure 2
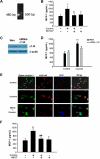
Engagement of σ-1R is critical for cocaine-induced MCP-1 expression in microglia. (A) RNA isolated from cocaine-treated BV-2 cells was subject to RT-PCR analysis using σ-1R primers. (B) Pretreatment of BV-2 cells with σ-1R antagonist BD1047 abolished cocaine-mediated induction of MCP-1 expression in BV-2 cells. (C) Whole-cell lysates from BV-2 cells transfected with either σ-1R or nonsense (Non) siRNAs were subject to Western blot analysis using antibodies specific for σ-1R. (D) σ-1R siRNA, but not Non siRNA, inhibited cocaine-mediated induction of MCP-1 expression. (E) BV-2 cells were treated with cocaine and double-stained using antibodies specific for ganglioside GM1-lipid raft marker (red TRITC fluorescence) or σ-1R (green FITC fluorescence). Overlay images are shown in the right panel. Data are representative from 3 typical experiments. Scale bars all indicate 20 μm. (F) Role of lipid rafts play in cocaine-mediated induction of MCP-1 in BV-2 cells. Cells were pretreated with MβCD (1mM) followed by treatment with cocaine. Supernatant fluids were harvested at 12 hours after cocaine treatment, followed by assessment of MCP-1 expression by ELISA. All the data are presented as means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05; ##P < .01 versus cocaine-treated group.
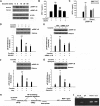
Figure 3
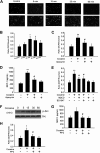
Cocaine-mediated induction of MCP-1 involves generation of ROS and Src kinase activation. (A) Cocaine-induced ROS generation in a time-dependent manner measured by laser-scanning microscopy. (B) BV-2 cells were treated with 10μM cocaine for the indicated time points (0-60 minutes) before incubation with carboxy-H2-DCF-DA and assessed for oxidative stress. Values are displayed as a ratio of the DCF fluorescent value to the Hoechst (nuclear stain) fluorescent value. A respiratory burst culminates after 15 minutes of stimulation. (C) BV-2 cells pretreated with apocynin (250μM) followed by stimulation with the cocaine for 30 minutes. Apocynin pretreatment resulted in abrogation of cocaine-induced respiratory burst. (D) Inhibition of NADPH oxidase by apocynin resulted in abrogation of cocaine-mediated induction of MCP-1. (E) Pretreatment of BV-2 cells with BD1047 and MβCD abrogated cocaine-induced ROS production. (F) Cocaine-induced Src phosphorylation in BV-2 cells. (G) Pretreatment with PP2 abrogated cocaine-induced ROS production. (H) Inhibition of the Src activity by Src inhibitor PP2 resulted in amelioration of cocaine-mediated induction of MCP-1. All the data are presented as means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05 versus cocaine-treated group.
Figure 4
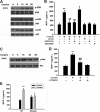
Cocaine-mediated induction of MCP-1 expression involves MAPKs and PI3K/Akt cell-signaling pathways. (A) Western blot analysis of time-dependent activation of ERK, JNK, and p38 by cocaine. (B) Inhibition of the ERK and JNK pathways by MEK1/2 (U0126) and JNK inhibitor (SP600125) resulted in amelioration of cocaine-mediated induction of MCP-1. (C) Time-dependent activation of Akt in cocaine-treated BV-2 cells. (D) Pretreatment with PI3K inhibitor (LY294002) resulted in inhibition of cocaine-mediated induction of MCP-1 expression. (E) Transduction with DN-Akt and not WT-Akt resulted in abrogation of cocaine-mediated induction of MCP-1. All the data are presented as means ± SD of 4 individual experiments. **P < .01 versus control group; ##P < .01 versus cocaine-treated group.
Figure 5
Involvement of σ-1R, Src kinase, and NADPH oxidase in the regulation of MAPKs and PI3K/Akt cell-signaling pathways. Pretreatment of BV-2 cells with σ-1R antagonist-BD1047 (A), σ-1R siRNA (B), Src inhibitor PP2 (C), or NADPH inhibitor apocynin (D) resulted in inhibition of cocaine-mediated phosphorylation of ERK, JNK, and Akt pathways. Representative immunoblots and the densitometric analyses of pERK/ERK, pJNK/JNK, and p-Akt/Akt from 4 separate experiments are presented. All the data are indicated as means ± SD of 4 individual experiments. **P < .01; ***P < .001 versus control group; #P < .05; ##P < .01; ###P < .001 versus cocaine-treated group.
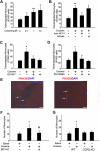
Figure 6
Cocaine-mediated induction of MCP-1 expression involves NF-κB activation. (A) Exposure of BV-2 cells to cocaine resulted in time-dependent increase in phosphorylation of the p65 subunit of NF-κB in the nuclear fraction, with a concomitant decrease in the cytosolic fraction. Reciprocally, cocaine exposure resulted in increased phosphorylation of IκBα in the cytosolic fraction of BV-2 cells. (B) Pretreatment with the IκBα inhibitor SC514 resulted in inhibition of cocaine-mediated induction of MCP-1. (C) Overexpression of the mutant but not the full-length p65/RelA NF-κB construct resulted in abrogation of cocaine-mediated induction of MCP-1. BV-2 cells exposed to cocaine in the presence or absence of σ-1R antagonist BD1047 (D), σ-1R siRNA (E), Src inhibitor PP2 (F), or NADPH inhibitor apocynin (G) were examined for cocaine-mediated translocation of NF-κB. Representative immunoblots and the densitometric analysis of p-P65 NF-κB/histone from 4 separate experiments are presented. All the data are means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05; ##P < .01 versus cocaine-treated group. (H) Schematic illustration of NF-κB binding consensus sequence on the MCP-1 promoter region. (I) ChIP assay demonstrating cocaine-mediated binding of p65NF-κB to the MCP-1 promoter. The image is representative of 3 independent experiments.
Figure 7
Cocaine-mediated induction of MCP-1 enhances monocyte transmigration both in vitro and in vivo. (A) Concentration-dependent transmigration of monocytes in the presence of CM from cocaine-treated BV-2 cells. (B) Increased monoctye transmigration in the presence of CM from cocaine-treated cells was ameliorated by the MCP-1–blocking antibody (1 μg/mL). (C) Pretreatment with BD1047 ameliorated cocaine-mediated increase in monoctye transmigration. (D) Pretreatment of monocytes with CCR2 antagonist RS102895 ameliorated cocaine-mediated increase in monoctye transmigration. All the data are presented as means ± SD of 4 individual experiments. *P < .05; **P < .01 versus control group; #P < .05 versus cocaine-treated group. (E) Detection of PKH26-labeled monocytes in the brains of mice treated with cocaine. Fluorescence micrographs show monocytes in the perivascular cuff (left panel; arrows) and the parenchymal (right panel; arrows) areas of the brain. (F) Increased monoctye transmigration observed in the cocaine-treated group was ameliorated by pretreatment of mice with BD1047. (G) Increased monocyte transmigration in the cocaine-treated WT but not CCR2 KO mice. *P < .05 versus saline group; #P < .05 versus cocaine group counted from the parenchyma. (F-G) Data are expressed as the mean number of PKH26-labeled cells in the entire area of 3 coronal brain sections ± SD (n = 6 per group).
Similar articles
- Cocaine hijacks σ1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS.
Yao H, Kim K, Duan M, Hayashi T, Guo M, Morgello S, Prat A, Wang J, Su TP, Buch S. Yao H, et al. J Neurosci. 2011 Apr 20;31(16):5942-55. doi: 10.1523/JNEUROSCI.5618-10.2011. J Neurosci. 2011. PMID: 21508219 Free PMC article. - Platelet-derived growth factor (PDGF)-BB-mediated induction of monocyte chemoattractant protein 1 in human astrocytes: implications for HIV-associated neuroinflammation.
Bethel-Brown C, Yao H, Hu G, Buch S. Bethel-Brown C, et al. J Neuroinflammation. 2012 Dec 1;9:262. doi: 10.1186/1742-2094-9-262. J Neuroinflammation. 2012. PMID: 23198981 Free PMC article. - LPS-induced MCP-1 expression in human microvascular endothelial cells is mediated by the tyrosine kinase, Pyk2 via the p38 MAPK/NF-kappaB-dependent pathway.
Anand AR, Bradley R, Ganju RK. Anand AR, et al. Mol Immunol. 2009 Feb;46(5):962-8. doi: 10.1016/j.molimm.2008.09.022. Epub 2008 Oct 26. Mol Immunol. 2009. PMID: 18954908 Free PMC article. - Role of Sigma-1 Receptor in Cocaine Abuse and Neurodegenerative Disease.
Cai Y, Yang L, Niu F, Liao K, Buch S. Cai Y, et al. Adv Exp Med Biol. 2017;964:163-175. doi: 10.1007/978-3-319-50174-1_12. Adv Exp Med Biol. 2017. PMID: 28315271 Review. - Monocyte chemoattractant protein-1 (MCP-1): an overview.
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Deshmane SL, et al. J Interferon Cytokine Res. 2009 Jun;29(6):313-26. doi: 10.1089/jir.2008.0027. J Interferon Cytokine Res. 2009. PMID: 19441883 Free PMC article. Review.
Cited by
- Involvement of sigma-1 receptor in astrocyte activation induced by methamphetamine via up-regulation of its own expression.
Zhang Y, Lv X, Bai Y, Zhu X, Wu X, Chao J, Duan M, Buch S, Chen L, Yao H. Zhang Y, et al. J Neuroinflammation. 2015 Feb 17;12:29. doi: 10.1186/s12974-015-0250-7. J Neuroinflammation. 2015. PMID: 25889537 Free PMC article. - Effects of Drugs of Abuse on the Blood-Brain Barrier: A Brief Overview.
Pimentel E, Sivalingam K, Doke M, Samikkannu T. Pimentel E, et al. Front Neurosci. 2020 May 21;14:513. doi: 10.3389/fnins.2020.00513. eCollection 2020. Front Neurosci. 2020. PMID: 32670001 Free PMC article. Review. - Cocaine Induces Inflammatory Gut Milieu by Compromising the Mucosal Barrier Integrity and Altering the Gut Microbiota Colonization.
Chivero ET, Ahmad R, Thangaraj A, Periyasamy P, Kumar B, Kroeger E, Feng D, Guo ML, Roy S, Dhawan P, Singh AB, Buch S. Chivero ET, et al. Sci Rep. 2019 Aug 21;9(1):12187. doi: 10.1038/s41598-019-48428-2. Sci Rep. 2019. PMID: 31434922 Free PMC article. - The cellular basis of fetal endoplasmic reticulum stress and oxidative stress in drug-induced neurodevelopmental deficits.
Tsai SA, Bendriem RM, Lee CD. Tsai SA, et al. Neurobiol Stress. 2018 Dec 27;10:100145. doi: 10.1016/j.ynstr.2018.100145. eCollection 2019 Feb. Neurobiol Stress. 2018. PMID: 30937351 Free PMC article. Review. - CB2 receptor agonists protect human dopaminergic neurons against damage from HIV-1 gp120.
Hu S, Sheng WS, Rock RB. Hu S, et al. PLoS One. 2013 Oct 17;8(10):e77577. doi: 10.1371/journal.pone.0077577. eCollection 2013. PLoS One. 2013. PMID: 24147028 Free PMC article.
References
- Dietrich JB. Alteration of blood-brain barrier function by methamphetamine and cocaine. Cell Tissue Res. 2009;336(3):385–392. - PubMed
- Goodkin K, Shapshak P, Metsch LR, et al. Cocaine abuse and HIV-1 infection: epidemiology and neuropathogenesis. J Neuroimmunol. 1998;83(1-2):88–101. - PubMed
- Zhang L, Looney D, Taub D, et al. Cocaine opens the blood-brain barrier to HIV-1 invasion. J Neurovirol. 1998;4(6):619–626. - PubMed
- Fiala M, Gan XH, Zhang L, et al. Cocaine enhances monocyte migration across the blood-brain barrier: cocaine's connection to AIDS dementia and vasculitis? Adv Exp Med Biol. 1998;437:199–205. - PubMed
- Fiala M, Eshleman AJ, Cashman J, et al. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J Neurovirol. 2005;11(3):281–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA024442/DA/NIDA NIH HHS/United States
- DA020392/DA/NIDA NIH HHS/United States
- MH-068212/MH/NIMH NIH HHS/United States
- R01 DA027729/DA/NIDA NIH HHS/United States
- DA023397/DA/NIDA NIH HHS/United States
- DA027729/DA/NIDA NIH HHS/United States
- R01 DA020392/DA/NIDA NIH HHS/United States
- R21 DA023397/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- DA024442/DA/NIDA NIH HHS/United States
- R01 MH068212/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous