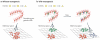New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? - PubMed (original) (raw)
Review
New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory?
Wei Deng et al. Nat Rev Neurosci. 2010 May.
Abstract
The integration of adult-born neurons into the circuitry of the adult hippocampus suggests an important role for adult hippocampal neurogenesis in learning and memory, but its specific function in these processes has remained elusive. In this article, we summarize recent progress in this area, including advances based on behavioural studies and insights provided by computational modelling. Increasingly, evidence suggests that newborn neurons might be involved in hippocampal functions that are particularly dependent on the dentate gyrus, such as pattern separation. Furthermore, newborn neurons at different maturation stages may make distinct contributions to learning and memory. In particular, computational studies suggest that, before newborn neurons are fully mature, they might function as a pattern integrator by introducing a degree of similarity to the encoding of events that occur closely in time.
Figures
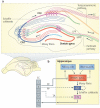
Figure 1. The neural circuitry in the rodent hippocampus
a | An illustration of the hippocampal circuitry. b | Diagram of the hippocampal neural network. The traditional excitatory trisynaptic pathway (entorhinal cortex (EC)–dentate gyrus–CA3–CA1–EC) is depicted by solid arrows. The axons of layer II neurons in the entorhinal cortex project to the dentate gyrus through the perforant pathway (PP), including the lateral perforant pathway (LPP) and medial perforant pathway (MPP). The dentate gyrus sends projections to the pyramidal cells in CA3 through mossy fibres. CA3 pyramidal neurons relay the information to CA1 pyramidal neurons through Schaffer collaterals. CA1 pyramidal neurons send back-projections into deep-layer neurons of the EC. CA3 also receives direct projections from EC layer II neurons through the PP. CA1 receives direct input from EC layer III neurons through the temporoammonic pathway (TA). The dentate granule cells also project to the mossy cells in the hilus and hilar interneurons, which send excitatory and inhibitory projections, respectively, back to the granule cells.
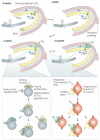
Figure 2. Adult hippocampal neurogenesis
The proliferation of neural progenitor cells (NPCs) with two different morphologies gives rise to adult-born dentate granule cells (DGCs) (shown in green). The fate-committed, adult-born DGCs undergo several stages of development, with gradual changes in morphological and physiological characteristics. About 1 week after birth, the adult-born DGC extends its dendrite into the granule cell layer (GCL) and molecular layer (MOL) and projects the axon into the hilus toward CA3. The DGC receives excitatory GABA (γ-aminobutyric acid)-ergic input, presumably from local interneurons (shown as blue cells). During the third week after birth, the DGC receives glutamatergic input (Glu) from the perforant pathway. At this stage, the GABA input changes from being excitatory to being inhibitory. Both efferent and afferent synapses of the adult-born DGCs begin to form around this time-. At around 2 months of age, the basic structural and physiological properties of the adult-born DGCs are indistinguishable from those of mature DGCs. The inset panels illustrate the competitive nature of synapse formation-. Left inset: a small bouton (shown in green) from the axon of an adult-born DGC contacts the dendritic shaft (shown in grey) of a CA3 pyramidal neuron at a site near the thorny excrescences that contact an existing axonal bouton (shown in yellow). During the subsequent development of the new synapse, the bouton from the newborn DGC either replaces the existing axonal bouton or forms a new thorny excrescence nearby, or retracts. Right inset: the filopodium (shown in green) from an adult-born DGC dendrite extends to an axonal bouton (shown in red) that is associated with another spine (shown in yellow), which leads to the eventual formation of either a monosynaptic bouton targeting spines from the adult-born DGC or a multisynaptic bouton, or leads to retraction.
Figure 3. Computational theories of neurogenesis
a | Without neurogenesis, new events (represented by different shapes) are limited by the set of sparse ‘codes’ (combinations of active neurons) provided by mature granule cells in the dentate gyrus. This can lead to the dentate gyrus not having the flexibility to encode new memories well, or to interference between memories formed in the hippocampus (shown as a cluster of memories in a projection of the high-dimension hippocampal ‘memory space’). b | New neurons (shown in green) provide new sparse codes for encoding new information, while older memories are preserved because they are represented by older neurons (shown in red). This can facilitate the formation of new memories while avoiding catastrophic interference, saving older memories- (shown in the left panel as two separate clusters of memories in a projection of the high-dimension hippocampal memory space). The three-way arrow indicates that new neurons can change how memories are encoded in the hippocampal network. Neurons born at different times (shown in green and blue in the right panel) represent different inputs, and the sparse codes generated at a particular time are clustered together (active neurons in a population are similar in composition to one another), separately from sparse codes that were generated at a different time, essentially encoding time into new memories,,.
Similar articles
- A new chapter in the field of memory: adult hippocampal neurogenesis.
Koehl M, Abrous DN. Koehl M, et al. Eur J Neurosci. 2011 Mar;33(6):1101-14. doi: 10.1111/j.1460-9568.2011.07609.x. Eur J Neurosci. 2011. PMID: 21395854 Review. - Involvement of Adult Hippocampal Neurogenesis in Learning and Forgetting.
Yau SY, Li A, So KF. Yau SY, et al. Neural Plast. 2015;2015:717958. doi: 10.1155/2015/717958. Epub 2015 Aug 25. Neural Plast. 2015. PMID: 26380120 Free PMC article. Review. - Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses.
Bruel-Jungerman E, Rampon C, Laroche S. Bruel-Jungerman E, et al. Rev Neurosci. 2007;18(2):93-114. doi: 10.1515/revneuro.2007.18.2.93. Rev Neurosci. 2007. PMID: 17593874 Review. - Krüppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis.
Scobie KN, Hall BJ, Wilke SA, Klemenhagen KC, Fujii-Kuriyama Y, Ghosh A, Hen R, Sahay A. Scobie KN, et al. J Neurosci. 2009 Aug 5;29(31):9875-87. doi: 10.1523/JNEUROSCI.2260-09.2009. J Neurosci. 2009. PMID: 19657039 Free PMC article. - Adult neurogenesis: integrating theories and separating functions.
Aimone JB, Deng W, Gage FH. Aimone JB, et al. Trends Cogn Sci. 2010 Jul;14(7):325-37. doi: 10.1016/j.tics.2010.04.003. Epub 2010 May 12. Trends Cogn Sci. 2010. PMID: 20471301 Free PMC article. Review.
Cited by
- Effect of adult hippocampal neurogenesis on pattern separation and its applications.
Wang Z, Yang K, Sun X. Wang Z, et al. Cogn Neurodyn. 2024 Oct;18(5):1-14. doi: 10.1007/s11571-024-10110-3. Epub 2024 Apr 16. Cogn Neurodyn. 2024. PMID: 39568526 - Spatially resolved transcriptomic signatures of hippocampal subregions and _Arc_-expressing ensembles in active place avoidance memory.
Vingan I, Phatarpekar S, Tung VSK, Hernández AI, Evgrafov OV, Alarcon JM. Vingan I, et al. Front Mol Neurosci. 2024 Oct 31;17:1386239. doi: 10.3389/fnmol.2024.1386239. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39544521 Free PMC article. - A Systematic Review of the Role of Gummosin in Improving Memory in the Scopolamine Impaired Memory Model.
Asadi Rizi A, Amjad L, Shahrani M, Amini Khoei H. Asadi Rizi A, et al. Arch Razi Inst. 2024 Apr 30;79(2):248-263. doi: 10.32592/ARI.2024.79.2.248. eCollection 2024 Apr. Arch Razi Inst. 2024. PMID: 39463714 Free PMC article. Review. - Post-stroke hippocampal neurogenesis is impaired by microvascular dysfunction and PI3K signaling in cerebral amyloid angiopathy.
Osborne OM, Daftari M, Naranjo O, Johar AN, Brooks S, Colbert BM, Torices S, Lewis E, Sendaydiego J, Drexler G, Bashti M, Margetts AV, Tuesta LM, Mason C, Bilbao D, Vontell R, Griswold AJ, Dykxhoorn DM, Toborek M. Osborne OM, et al. Cell Rep. 2024 Oct 22;43(10):114848. doi: 10.1016/j.celrep.2024.114848. Epub 2024 Oct 10. Cell Rep. 2024. PMID: 39392753 Free PMC article. - Beneficial Effect of Dimethyl Fumarate Drug Repositioning in a Mouse Model of TDP-43-Dependent Frontotemporal Dementia.
Silva-Llanes I, Martín-Baquero R, Berrojo-Armisen A, Rodríguez-Cueto C, Fernández-Ruiz J, De Lago E, Lastres-Becker I. Silva-Llanes I, et al. Antioxidants (Basel). 2024 Sep 2;13(9):1072. doi: 10.3390/antiox13091072. Antioxidants (Basel). 2024. PMID: 39334731 Free PMC article.
References
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. - PubMed
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. - PubMed
- Enikolopov G, Overstreet Wadiche L. In: Adult Neurogenesis. Gage FH, Kempermann G, Song H, editors. Cold Spring Harbor Laboratory Press; New York: 2008. pp. 81–100.
- Kuhn HG, Peterson DA. In: Adult Neurogenesis. Gage FH, Kempermann G, Song H, editors. Cold Spring Harbor Laboratory Press; New York: 2008. pp. 25–47.
- Zhao C. In: Adult Neurogenesis. Gage FH, Kempermann G, Song H, editors. Cold Spring Harbor Laboratory Press; New York: 2008. pp. 101–117.
Publication types
MeSH terms
Grants and funding
- R01 NS050217-05/NS/NINDS NIH HHS/United States
- R01 AG020938/AG/NIA NIH HHS/United States
- R01 AG020938-05/AG/NIA NIH HHS/United States
- NS-050217/NS/NINDS NIH HHS/United States
- AG-020938/AG/NIA NIH HHS/United States
- R01 NS050217/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical