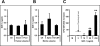Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex - PubMed (original) (raw)
Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex
M Z Ratajczak et al. Leukemia. 2010 May.
Abstract
The complement cascade (CC) becomes activated and its cleavage fragments play a crucial role in the mobilization of hematopoietic stem/progenitor cells (HSPCs). Here, we sought to determine which major chemoattractant present in peripheral blood (PB) is responsible for the egress of HSPCs from the bone marrow (BM). We noticed that normal and mobilized plasma strongly chemoattracts HSPCs in a stromal-derived factor-1 (SDF-1)-independent manner because (i) plasma SDF-1 level does not correlate with mobilization efficiency; (ii) the chemotactic plasma gradient is not affected in the presence of AMD3100 and (iii) it is resistant to denaturation by heat. Surprisingly, the observed loss of plasma chemotactic activity after charcoal stripping suggested the involvement of bioactive lipids and we focused on sphingosine-1-phosphate (S1P), a known chemoattracant of HSPCs. We found that S1P (i) creates in plasma a continuously present gradient for BM-residing HSPCs; (ii) is at physiologically relevant concentrations a chemoattractant several magnitudes stronger than SDF-1 and (iii) its plasma level increases during mobilization due to CC activation and interaction of the membrane attack complex (MAC) with erythrocytes that are a major reservoir of S1P. We conclude and propose a new paradigm that S1P is a crucial chemoattractant for BM-residing HSPCs and that CC through MAC induces the release of S1P from erythrocytes for optimal egress/mobilization of HSPCs.
Figures
Figure 1. Plasma SDF-1 level does not correlate with mobilization efficiency of HSPCs
Panel A - SDF-1 concentrations are low (less than 1 ng/ml) and do not differ significantly in the plasma of patients who are poor (n=7 or good (n=9) G-CSF mobilizers Panel B - As in patients, SDF-1 concentration is murine plasma is also low (less than 2 ng/ml) and does not increase significantly during Zymosan- or G-CSF-induced mobilization. Panel C – SDF-1 at a physiological concentration does not show chemotactic activity against murine BM HSPCs. The data shown in Panels B and C represent the combined results from three independent experiments carried out in triplicate per group (n=9). * p<0.05, ** p<0.01
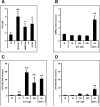
Figure 2. HSPCs egress from BM into PB in an SDF-1-independent manner
Panel A – Murine CFU-GM are much more efficiently chemoattracted by murine plasma than by a physiological concentration of SDF-1. Potential involvement of SDF-1 can be excluded because normal CFU-GM progenitor cells (black bar) responded to heat-inactivated plasma (HI-P) as well as the presence of 5 μM AMD3100 (white bar - NP). However, exposure to AMD3100-inhibited chemotaxis to SDF-1. Panel B - CFU-GM cells do not migrate to charcoal-stripped, lipid-free plasma; however they respond normally to the SDF-1 added (50 ng/ml) charcoal-stripped plasma. M, media-alone control; NP, normal murine plasma (2.5%); HI-P, heat-inactivated murine plasma (2.5%); SDF-1 (ng/ml); FP, filtered normal plasma filtered with 0.22 μm membrane; FP-LF, charcoal-stripped and then filtered plasma. * p<0.01. The data shown are the combined results from three independent experiments carried out in triplicate per group (n=9).
Figure 3. S1P is a major chemotactic component of plasma
Panel A - Murine plasma S1P levels increase during mobilization (10 min after zymosan-, 1 h after AMD3100-, and 6 h at day 6 after the last G-CSF-treatment). Panel B and C - However, S1P does not chemoattract differentiated/mature BMNCs; (B), it strongly chemoattracts BM-residing naïve HSPCs (C). Panel D – S1P has much lower chemotactic activity against G-CSF-mobilized PB circulating HSPCs. Ctl, normal mouse plasma; M, media only control; SDF-1 (ng/ml). * p<0.01, ** p<0.001. The data shown represent the combined results from three independent experiments carried out in triplicate per group (n=9).
Figure 4. S1P exposed- or plasma pre-incubated BM-HSPCs do not respond to S1P
Pre-incubation of BM-HSPCs with plasma from the same donor (Panel A, striped bar) or with S1P (Panel B), gray bar-100 nM, white bar-500 nM) for 1 h abrogated migration of BM-naïve CFU-GM progenitors to an S1P gradient. M, media alone control. * p<0.001. The data shown represent the combined results from three independent experiments carried out in triplicate per group (n=9).
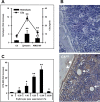
Figure 5. The role of MAC in mobilization
Panel A – Free hemoglobin level in plasma correlates with strength of CC activation. Panel B – MAC deposits are detectable in BM of G-CSF-mobilized wt but not C5−/− mice. Diffuse MAC deposition is visible in endothelial cells (arrow), osteoblasts (arrow head), and interstitium. Original magnification ×200. Panel C – Chemotactic response of BM CFU-GM to erythrocyte lysates prepared from plasma-washed erythrocytes followed by repeated freezing and thawing. Ctl, normal mouse plasma; M, media only control. * p<0.01, ** p<0.001. The data shown represent the combined results from three independent experiments carried out in triplicate per group (n=9).
Figure 6. Impaired responsiveness of HSPCs from DOP- treated mice to S1P
Panel A - BM HSPCs harvested from DOP- treated mice (white bar) do not migrate to a S1P gradient. Panel B - D - DOP treated mice are poor AMD3100 mobilizers *p<0.0001. The data shown represent the combined results from three independent experiments carried out in triplicate per group (n=9) and the combined results of three independent mobilization experiments carried out with 5 mice per group (n=15).
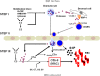
Figure 7. Novel mechanistic insight into BM HSPC mobilization
HSPCs are actively retained in BM and retention signals in the BM niches counteract a S1P-mediated chemotactic plasma gradient. Mobilizing agents disrupt major HSPC-anchoring signals (i.e., CXCR4-SDF-1 and VLA4-VCAM-1 interactions) and release HSPCs from their niches (Step I). First, as reported previously,, activated granulocytes egress from BM into sinusoids in response to C5 cleavage fragments and thus pave the way for HSPC (Step II). Next, MAC generated in the final step of CC activation enhances release of S1P from erythrocytes into the plasma, and the plasma S1P level directs egress of HSPCs into PB (Step III).
Similar articles
- S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release.
Golan K, Vagima Y, Ludin A, Itkin T, Cohen-Gur S, Kalinkovich A, Kollet O, Kim C, Schajnovitz A, Ovadya Y, Lapid K, Shivtiel S, Morris AJ, Ratajczak MZ, Lapidot T. Golan K, et al. Blood. 2012 Mar 15;119(11):2478-88. doi: 10.1182/blood-2011-06-358614. Epub 2012 Jan 25. Blood. 2012. PMID: 22279055 Free PMC article. - Sphingosine-1-phosphate-mediated mobilization of hematopoietic stem/progenitor cells during intravascular hemolysis requires attenuation of SDF-1-CXCR4 retention signaling in bone marrow.
Mierzejewska K, Klyachkin YM, Ratajczak J, Abdel-Latif A, Kucia M, Ratajczak MZ. Mierzejewska K, et al. Biomed Res Int. 2013;2013:814549. doi: 10.1155/2013/814549. Epub 2013 Dec 29. Biomed Res Int. 2013. PMID: 24490172 Free PMC article. - An emerging link in stem cell mobilization between activation of the complement cascade and the chemotactic gradient of sphingosine-1-phosphate.
Ratajczak MZ, Borkowska S, Ratajczak J. Ratajczak MZ, et al. Prostaglandins Other Lipid Mediat. 2013 Jul-Aug;104-105:122-9. doi: 10.1016/j.prostaglandins.2012.07.003. Epub 2012 Sep 3. Prostaglandins Other Lipid Mediat. 2013. PMID: 22981511 Free PMC article. Review. - Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells.
Lee H, Ratajczak MZ. Lee H, et al. Arch Immunol Ther Exp (Warsz). 2009 Jul-Aug;57(4):269-78. doi: 10.1007/s00005-009-0037-6. Epub 2009 Jul 4. Arch Immunol Ther Exp (Warsz). 2009. PMID: 19578812 Review.
Cited by
- Bioactive lipids S1P and C1P are prometastatic factors in human rhabdomyosarcoma, and their tissue levels increase in response to radio/chemotherapy.
Schneider G, Bryndza E, Abdel-Latif A, Ratajczak J, Maj M, Tarnowski M, Klyachkin YM, Houghton P, Morris AJ, Vater A, Klussmann S, Kucia M, Ratajczak MZ. Schneider G, et al. Mol Cancer Res. 2013 Jul;11(7):793-807. doi: 10.1158/1541-7786.MCR-12-0600. Epub 2013 Apr 24. Mol Cancer Res. 2013. PMID: 23615526 Free PMC article. - Hematopoietic stem cell mobilization: updated conceptual renditions.
Bonig H, Papayannopoulou T. Bonig H, et al. Leukemia. 2013 Jan;27(1):24-31. doi: 10.1038/leu.2012.254. Epub 2012 Sep 6. Leukemia. 2013. PMID: 22951944 Free PMC article. Review. - The SphKs/S1P/S1PR1 axis in immunity and cancer: more ore to be mined.
Jin L, Liu WR, Tian MX, Fan J, Shi YH. Jin L, et al. World J Surg Oncol. 2016 Apr 29;14:131. doi: 10.1186/s12957-016-0884-7. World J Surg Oncol. 2016. PMID: 27129720 Free PMC article. Review. - Markers of Regenerative Processes in Patients with Bipolar Disorder: A Case-control Study.
Reginia A, Samochowiec J, Jabłoński M, Ferensztajn-Rochowiak E, Rybakowski JK, Telesiński A, Tarnowski M, Misiak B, Ratajczak MZ, Kucharska-Mazur J. Reginia A, et al. Brain Sci. 2020 Jun 30;10(7):408. doi: 10.3390/brainsci10070408. Brain Sci. 2020. PMID: 32629800 Free PMC article. - Innate immunity and the regulation and mobilization of keratinocyte stem cells: are the old players playing a new game?
Singh A, Morris RJ. Singh A, et al. Exp Dermatol. 2012 Sep;21(9):660-4. doi: 10.1111/j.1600-0625.2012.01566.x. Exp Dermatol. 2012. PMID: 22897573 Free PMC article. Review.
References
- Lee H, Ratajczak MZ. Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells. Arch Immunol Ther Exp (Warsz) 2009;57(4):269–278. - PubMed
- Kassirer M, Zeltser D, Gluzman B, Leibovitz E, Goldberg Y, Roth A, et al. The appearance of L-selectin(low) polymorphonuclear leukocytes in the circulating pool of peripheral blood during myocardial infarction correlates with neutrophilia and with the size of the infarct. Clin Cardiol. 1999;22(11):721–726. - PMC - PubMed
- Kyne L, Hausdorff JM, Knight E, Dukas L, Azhar G, Wei JY. Neutrophilia and congestive heart failure after acute myocardial infarction. Am Heart J. 2000;139(1Pt1):94–100. - PubMed
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK074720/DK/NIDDK NIH HHS/United States
- R01 DK074720-05/DK/NIDDK NIH HHS/United States
- RC1 HL099447/HL/NHLBI NIH HHS/United States
- 1RC1 HL099447/HL/NHLBI NIH HHS/United States
- R01 CA106281/CA/NCI NIH HHS/United States
- XE00025/CAPMC/ CIHR/Canada
- R01 CA106281-05/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases