Transgenic rat model of neurodegeneration caused by mutation in the TDP gene - PubMed (original) (raw)
Transgenic rat model of neurodegeneration caused by mutation in the TDP gene
Hongxia Zhou et al. PLoS Genet. 2010.
Abstract
TDP-43 proteinopathies have been observed in a wide range of neurodegenerative diseases. Mutations in the gene encoding TDP-43 (i.e., TDP) have been identified in amyotrophic lateral sclerosis (ALS) and in frontotemporal lobe degeneration associated with motor neuron disease. To study the consequences of TDP mutation in an intact system, we created transgenic rats expressing normal human TDP or a mutant form of human TDP with a M337V substitution. Overexpression of mutant, but not normal, TDP caused widespread neurodegeneration that predominantly affected the motor system. TDP mutation reproduced ALS phenotypes in transgenic rats, as seen by progressive degeneration of motor neurons and denervation atrophy of skeletal muscles. This robust rat model also recapitulated features of TDP-43 proteinopathies including the formation of TDP-43 inclusions, cytoplasmic localization of phosphorylated TDP-43, and fragmentation of TDP-43 protein. TDP transgenic rats will be useful for deciphering the mechanisms underlying TDP-43-related neurodegenerative diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
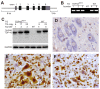
Figure 1. Postnatal death in transgenic founder rats constitutively expressing a mutant human TDP transgene.
(A) Schematic diagram shows the structure of the human TDP transgene extracted from a BAC clone. The M337V mutation was introduced into the TDP transgene construct using a recombineering technique. (B) PCR identified transgenic founders carrying the normal (WT) or mutant (TDP43M337V) human TDP gene. NT: nontransgenic littermate. (C) Immunoblotting detected a robust expression of the TDP transgene in the forebrain of TDP43M337V transgenic founders and TDP43WT transgenic offspring (first generation). Membranes were probed with an antibody against human TDP-43 (generated in-house) and then with an antibody against GAPDH. NT: a nontransgenic littermate. (D–F) Immunohistochemistry revealed that human TDP-43 was expressed in the normal (E) and mutant (F) human TDP transgenic rats, but not in nontransgenic littermates (D) at the age of 29 days. Transverse sections through the L4 spinal cord were stained for human TDP-43 immunoreactivity and then counterstained with haematoxylin. Micrographs show the ventral horn of the lumbar spinal cord.
Figure 2. Progressive paralysis in transgenic rats conditionally expressing a mutant human TDP gene.
(A) Schematic diagram shows the structure of the inducible mutant TDP transgene (TRE-TDP-43M337V). Expression of the mutant TDP transgene depends on tTA activation and can be suppressed by Dox, which binds to tTA and renders it inactive. (B) Immunoblotting detected a robust expression of the transgene in the spinal cord of P20 rats. Membranes were probed sequentially with antibodies against human TDP-43 (generated in-house), human and rat TDP-43 (ProteinTech), and rat GAPDH. M337V: transgenic rats carrying the conditional mutant TDP (TRE-TDP-43M337V) and CAG-tTA transgenes; WT: transgenic rat carrying the mini normal human TDP transgene (miniTDP-43WT); NT: nontransgenic littermate of the WT transgenic rat; *, a weak nonspecific band. (C) Photo of a mutant TDP transgenic rat (line 7) paralyzed at P40. (D) Graphs show the probability of disease onset, defined as an unrecoverable reduction in running time on a Rotarod. Disease onset for line 16 was not plotted, since these animals experienced early paralysis with rapid progression and accurate definition of disease onset was technically difficult. (E) Survival analysis revealed that lifespan was remarkably reduced in the mutant TDP transgenic rats. Rats were euthanized and counted as dead when two or more legs became paralyzed. Male and female rats of line 16 were combined as one group because disease progression between each gender was indistinguishable. Definition of symbols in (D,E): ▪, normal TDP transgenic rats carrying the miniTDP-43WT transgene (line 4; n = 9); • and ○, mutant male (•, n = 10) and female (○, n = 12) transgenic rats carrying the TRE-TDP-43M337V (line 7) and CAGtTA transgenes; ♦, mutant TDP transgenic rats carrying the TRE-TDP-43M337V (line 16) and the CAGtTA transgenes (n = 14). All breeding female rats were given Dox in drinking water (50 µg/ml) until 4 days before delivery.
Figure 3. Neuronal death and axonal damage in transgenic rats expressing a mutant human TDP gene.
(A–C) Transverse sections of lumbar spinal cord were immunostained for human TDP-43 (red) and ChAT (green). Scale bars: 30 µm. (D–F) Cresyl violet staining revealed motor neurons in the ventral horn of L3 spinal segments. Scale bars: 100 µm. (G–I) Toluidine blue staining shows axons of L3 ventral roots. (J,K) Transmission electron microscopy (EM) shows the structure of axons in the L3 ventral roots. Degenerating axons were shrunken and had collapsed myelin (arrows). Scale bars: 5 µm. (L) The number of large neurons (>25 µm in diameter) in the ventral horn of L3 spinal segments was estimated by stereological cell counting. Data are expressed as the mean ± SD (n = 9–15). *p<0.05. (M) Axons of L3 ventral roots were visualized by EM, and 60 axons (>4 µm in diameter) of each animal were examined for integrity. Data are expressed as mean ± SD (n = 8 or 9). *p<0.01. Tissues were collected from paralyzed transgenic rats of line 7 (age: 45±7 days) conditionally expressing the mutant human TDP gene (M337V: C, F, I, K, L, and M), age-matched nontransgenic littermates (NT: A, D, G, L, and M), and age-matched transgenic rats constitutively expressing the normal human mini TDP gene (WT: B, E, H, J, L, and M).
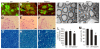
Figure 4. Denervation atrophy of skeletal muscle in transgenic rats expressing a mutant human TDP gene.
(A–C) The neuromuscular junction (NMJ) in the gastrocnemius muscles was examined by confocal microscopy to reconstruct the focal structure. Compared to the NMJ in a control rat (A), the NMJ in TRE-TDP-43M337V transgenic rats (line 7) was partially denervated at disease onset (B) and severely denervated at disease end stages (C). Axon terminals were visualized by immunostaining for synaptophysin and neurofilament (NF), while postsynaptic nicotinic receptors were visualized with Alexa fluor 555-conjugated α-bungarotoxin. (D) Quantification of NMJ denervation in nontransgenic control rats (NT) and TRE-TDP-43M337V transgenic rats at disease onset or the paralysis stage. Twenty NMJs were examined for each animal (n = 4 or 5). (E) Electromyography of the gastrocnemius muscles revealed that frequent fibrillation potentials (arrows) were present in paralyzed TRE-TDP-43M337V transgenic rats (M337V), but not in a nontransgenic littermate (NT). (F,G) EM revealed that intramuscular axons underwent degeneration in a mutant transgenic rat (line 7, age: 32 days) at disease onset (G), but not in an age-matched nontransgenic littermate (F). The arrow indicates a cluster of aggregates that accumulated in the axon of a mutant rat (G). SM: skeletal muscle; a: axon; N: the nucleus of a Schwann cell. Scale bars: 2 µm. (H,I) Group atrophy of the gastrocnemius muscle in paralyzed TRE-TDP-43M337V transgenic rats was detected by H&E staining (H, arrows) and by histochemistry for nonspecific esterase (I).
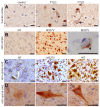
Figure 5. Degeneration of motor neurons in paralyzed mutant TDP transgenic rats.
(A,B) Bielschowski silver staining revealed degenerating neurons in the lumbar spinal cord of a paralyzed TRE-TDP-43M337V transgenic rat (line 7) (B), but not in the spinal cord of a nontransgenic littermate (A). (C,D) TUNEL staining shows motor neurons in the spinal cord undergoing apoptosis (D: arrow) in a paralyzed TRE-TDP-43M337V transgenic rat ( line 7) (D), but not in its nontransgenic littermate (C). Transverse sections of the L3 spinal segment were stained with ChAT antibody to visualize motor neurons and were labeled using a TUNEL staining kit to visualize apoptosis.
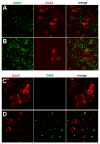
Figure 6. Activation of glial cells in paralyzed mutant TDP transgenic rats.
(A,B) Double immunofluorescence staining shows an accumulation of astrocytes around motor neurons in a paralyzed TRE-TDP-43M337V transgenic rat (line 7) (B), but not in a nontransgenic littermate (A). (C,D) Double immunofluorescence staining shows an activation of microglial cells in a paralyzed TRE-TDP-43M337V transgenic rat (line 7) (D), but not in a nontransgenic littermate (C). Transverse sections of the L3 spinal cord were immunostained for ChAT (red; motor neuron marker), GFAP (green; astrocyte marker), or cd68 (green; microglia and macrophage marker).
Figure 7. Degeneration of non-motor neurons in paralyzed mutant TDP transgenic rats.
(A–F) FD silver staining shows degeneration of non-motor neurons in paralyzed tTA/TRE-TDP-43M337V bigenic rats (line 7; age: 45 days) (B,D,F), but not in a tTA transgenic littermate (A,B,C). Degenerating neurons were outlined by deposits of silver particles (arrows). Scale bars: 20 µm.
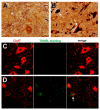
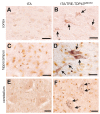
Figure 8. Phosphorylation and cytoplasmic localization of TDP-43 in FTLD patients and in TDP transgenic rats.
(A) Immunohistochemistry revealed phosphorylation and cytoplasmic localization of TDP-43 (arrows) in the brains of FTLD patients, but not in control subjects. Paraffin-embedded sections were stained with a polyclonal antibody against TDP-43 phosphorylated at aa409/410. (B,C) Immunostaining revealed that human TDP-43 was present in both the nucleus and cytoplasm of cells from the mutant (M337V: line 7) and normal (WT) TDP transgenic rats, but not in the cells of a nontransgenic rat (NT). TDP-43 inclusions (arrow) were rarely detected, being present in only the cortex of paralyzed M337V transgenic rats. Cryopreserved sections of the rat brain and spinal cord were stained with a polyclonal antibody specific to human TDP-43. (D) Immunostaining shows a cytoplasmic accumulation of phosphorylated TDP-43 in mutant (M337V) and normal (WT) TDP transgenic rats. A weak signal for phosphorylated TDP-43 was also detected in the nuclei of cells from a nontransgenic rat (NT). Cryopreserved sections of the spinal cord were stained with a polyclonal antibody against TDP-43 phosphorylated at aa409/410. All scale bars: 20 µm.
Similar articles
- Mutant TDP-43 in motor neurons promotes the onset and progression of ALS in rats.
Huang C, Tong J, Bi F, Zhou H, Xia XG. Huang C, et al. J Clin Invest. 2012 Jan;122(1):107-18. doi: 10.1172/JCI59130. Epub 2011 Dec 12. J Clin Invest. 2012. PMID: 22156203 Free PMC article. - Temporal Expression of Mutant TDP-43 Correlates with Early Amyotrophic Lateral Sclerosis Phenotype and Motor Weakness.
Chen Q, Zhou J, Huang C, Huang B, Bi F, Zhou H, Xiao B. Chen Q, et al. Curr Neurovasc Res. 2018;15(1):3-9. doi: 10.2174/1567202615666180109161541. Curr Neurovasc Res. 2018. PMID: 29313467 Free PMC article. - Single-copy expression of an amyotrophic lateral sclerosis-linked TDP-43 mutation (M337V) in BAC transgenic mice leads to altered stress granule dynamics and progressive motor dysfunction.
Gordon D, Dafinca R, Scaber J, Alegre-Abarrategui J, Farrimond L, Scott C, Biggs D, Kent L, Oliver PL, Davies B, Ansorge O, Wade-Martins R, Talbot K. Gordon D, et al. Neurobiol Dis. 2019 Jan;121:148-162. doi: 10.1016/j.nbd.2018.09.024. Epub 2018 Oct 2. Neurobiol Dis. 2019. PMID: 30290270 - The role of transactive response DNA-binding protein-43 in amyotrophic lateral sclerosis and frontotemporal dementia.
Mackenzie IR, Rademakers R. Mackenzie IR, et al. Curr Opin Neurol. 2008 Dec;21(6):693-700. doi: 10.1097/WCO.0b013e3283168d1d. Curr Opin Neurol. 2008. PMID: 18989115 Free PMC article. Review. - [Clinical and pathological spectrum of TDP-43 associated ALS].
Onodera O, Yokoseki A, Tan CF, Ishihara T, Nishiira Y, Toyoshima Y, Kakita A, Nishizawa M, Takahashi H. Onodera O, et al. Rinsho Shinkeigaku. 2010 Nov;50(11):940-2. doi: 10.5692/clinicalneurol.50.940. Rinsho Shinkeigaku. 2010. PMID: 21921519 Review. Japanese.
Cited by
- Wild type human TDP-43 potentiates ALS-linked mutant TDP-43 driven progressive motor and cortical neuron degeneration with pathological features of ALS.
Mitchell JC, Constable R, So E, Vance C, Scotter E, Glover L, Hortobagyi T, Arnold ES, Ling SC, McAlonis M, Da Cruz S, Polymenidou M, Tessarolo L, Cleveland DW, Shaw CE. Mitchell JC, et al. Acta Neuropathol Commun. 2015 Jun 25;3:36. doi: 10.1186/s40478-015-0212-4. Acta Neuropathol Commun. 2015. PMID: 26108367 Free PMC article. - Targeted depletion of TDP-43 expression in the spinal cord motor neurons leads to the development of amyotrophic lateral sclerosis-like phenotypes in mice.
Wu LS, Cheng WC, Shen CK. Wu LS, et al. J Biol Chem. 2012 Aug 10;287(33):27335-44. doi: 10.1074/jbc.M112.359000. Epub 2012 Jun 20. J Biol Chem. 2012. PMID: 22718760 Free PMC article. - The Progranulin Cleavage Products, Granulins, Exacerbate TDP-43 Toxicity and Increase TDP-43 Levels.
Salazar DA, Butler VJ, Argouarch AR, Hsu TY, Mason A, Nakamura A, McCurdy H, Cox D, Ng R, Pan G, Seeley WW, Miller BL, Kao AW. Salazar DA, et al. J Neurosci. 2015 Jun 24;35(25):9315-28. doi: 10.1523/JNEUROSCI.4808-14.2015. J Neurosci. 2015. PMID: 26109656 Free PMC article. - XBP1 depletion precedes ubiquitin aggregation and Golgi fragmentation in TDP-43 transgenic rats.
Tong J, Huang C, Bi F, Wu Q, Huang B, Zhou H. Tong J, et al. J Neurochem. 2012 Nov;123(3):406-16. doi: 10.1111/jnc.12014. J Neurochem. 2012. PMID: 22970712 Free PMC article. - ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43.
Arnold ES, Ling SC, Huelga SC, Lagier-Tourenne C, Polymenidou M, Ditsworth D, Kordasiewicz HB, McAlonis-Downes M, Platoshyn O, Parone PA, Da Cruz S, Clutario KM, Swing D, Tessarollo L, Marsala M, Shaw CE, Yeo GW, Cleveland DW. Arnold ES, et al. Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):E736-45. doi: 10.1073/pnas.1222809110. Epub 2013 Feb 4. Proc Natl Acad Sci U S A. 2013. PMID: 23382207 Free PMC article.
References
- Buratti E, Brindisi A, Giombi M, Tisminetzky S, Ayala MY, et al. TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: an important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing. J Biol Chem. 2005;280:37572–37584. - PubMed
- Abhyankar MM, Urekar C, Reddi PP. A novel CpG-free vertebrate insulator silences the testis-specific SP-10 gene in somatic tissues: role for TDP-43 in insulator function. J Biol Chem. 2007;282:36143–36154. - PubMed
- Wang YH, Wang FI, Bose J, Shen KC. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004;83:130–139. - PubMed
- Neumann M, Sampathu MD, Kwong KL, Truax CA, Micsenyi CM, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RR024586A/RR/NCRR NIH HHS/United States
- R21 RR024586-02/RR/NCRR NIH HHS/United States
- R01 ES016760/ES/NIEHS NIH HHS/United States
- R21 RR024586-01A1/RR/NCRR NIH HHS/United States
- R21 RR024586/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous