The primary cilium as a Hedgehog signal transduction machine - PubMed (original) (raw)
The primary cilium as a Hedgehog signal transduction machine
Sarah C Goetz et al. Methods Cell Biol. 2009.
Abstract
The Hedgehog (Hh) signal transduction pathway is essential for the development and patterning of numerous organ systems, and has important roles in a variety of human cancers. Genetic screens for mouse embryonic patterning mutants first showed a connection between mammalian Hh signaling and intraflagellar transport (IFT), a process required for construction of the primary cilium, a small cellular projection found on most vertebrate cells. Additional genetic and cell biological studies have provided very strong evidence that mammalian Hh signaling depends on the primary cilium. Here, we review the evidence that defines the integral roles that IFT proteins and cilia play in the regulation of the Hh signal transduction pathway in vertebrates. We discuss the mechanisms that control localization of Hh pathway proteins to the cilium, focusing on the transmembrane protein Smoothened (Smo), which moves into the cilium in response to Hh ligand. The phenotypes caused by loss of cilia-associated proteins are complex, which suggests that cilia and IFT play active roles in mediating Hh signaling rather than serving simply as a compartment in which pathway components are concentrated. Hh signaling in Drosophila does not depend on cilia, but there appear to be ancient links between cilia and components of the Hh pathway that may reveal how this fundamental difference between the Drosophila and mammalian Hh pathways arose in evolution.
2009 Elsevier Inc. All rights reserved.
Figures
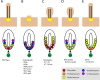
Figure 1. Summary of IFT mutants and their associated neural patterning defects
Upper row: schematic of cilia morphology; lower row, schematic of neural tube patterning in the same mutants. (A) In wild-type, cilia are required for cells to respond to Shh, which is essential for the specification of a set of cell types in the ventral neural tube. The notochord (dark green) located ventral to the neural tube acts as the initial source of the Shh signal (arrow). In the absence of Shh, none of the indicated ventral neural cell types are specified. (B) In mutants that lack the anterograde IFT motor Kinesin-II or components of the IFT-B complex, cilia are severely shortened or absent, the most Shh-dependent ventral neural cell fates, floor plate and V3 interneurons are absent, and the number of motor neurons is severely reduced (Huangfu et al., 2003; Houde et al., 2006; Liu, 2005). (C) IFT-A complex mutants have abnormal cilia with bulges and tend to be short. The neural tubes in these embryos show expansion of Hh-dependent ventral cell fates (Cortellino et al., 2009; Tran et al., 2008). (D) Dynein mutants, which are deficient in retrograde trafficking, have bulged cilia similar to those of IFT-A mutants, but their neural patterning phenotype is consistent with a loss of Hh signaling. Rostrally, the phenotype resembles that depicted in (B), while caudally motor neurons are specified (Huangfu and Anderson, 2005; May et al., 2005). (E) Arl13b hnn cilia have structural defects in the axoneme. Neural patterning in these mutants is unusual, with a loss of ventral-most cell fates, but an expansion of motor neurons, which depend of intermediate levels of Shh for their specification (Caspary et al., 2007). Cilia in Kif7 mutants appear normal, but motor neurons are expanded dorsally (not shown).
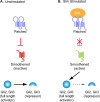
Figure 2. The core Hh signaling pathway
The vertebrate Hedgehog (Hh) pathway in the absence (A) or presence (B) of Sonic hedgehog (Shh) ligand. (A) In the absence of ligand, the Shh receptor Ptch1 prevents the activation of another transmembrane domain protein, Smo. In this state, full length Gli2 and Gli3 are proteolytically processed into a smaller repressor form. (B) Upon Shh ligand binding to Ptch1, the inhibition on Smo is relieved and activates full-length Gli proteins and blocks production of Gli repressors.
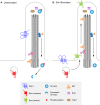
Figure 3. Localization of Hh pathway components to the primary cilium in the presence and absence of Shh
(A) In the absence of Shh ligand, Ptch1 is enriched in primary cilia and Smo is not. Sufu and Gli are present at the cilia tip, and events take place that promote proteolytic processing of Gli3 to the transcriptional repressor form (red star), causing repression of Hh target genes. KIf7, a kinesin homologous to Drosophila Cos2, is present at the base of the cilium and helps prevent activation of the pathway. (B) In the presence of Shh ligand, Shh binds to Ptch1, and Ptch1 moves out of the ciliary axoneme. In parallel, Smo is enriched in primary cilia and promotes activation rather than proteolytic processing of Gli proteins. Kif7 moves from the base to the tips of cilia and contributes to the activation of Gli proteins. Activated Gli proteins are transported out of the cilium to turn on Shh target gene expression in the nucleus. Sufu and Gli proteins are localized to the tip of the cilium in both the presence and absence of ligand, as is Tulp3.
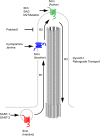
Figure 4. The components of anterograde and retrograde IFT complexes
Anterograde transport from the basal body to the distal tip is driven by the heterotrimeric Kinesin-II complex, composed of Kif3a, Kif3b (pink) and KAP3 (green), and the multiprotein IFT-B complex (red). At the tip of the cilium, IFT turnaround and remodeling of the IFT complexes occur in a poorly-understood process (Pedersen et al., 2006). Recycling of ciliary components to the base of the cilium is mediated by cytoplasmic dynein 2 (blue), together with the IFT-A complex (orange).
Figure 5. Smo activation requires localization to the primary cilium
Modified from Rohatgi et al. 2009. Smo is activated through a series of steps and conformations that can be stabilized by small molecule regulators of Hh signaling. Full activation of Smo requires the transport of inactive cytoplasmic Smo (red) from the cytoplasm into the cilium controlled by step R1. The Smo antagonists SANT-1 and -2 stabilize Smo in this inactive state. Smo within the cilium can exist in an inactive state (blue) that is trafficked out of the cilium when no further activation is achieved (R3). This inactive form is stabilized by the antagonist cyclopamine. Smo can be activated (green) through a second step, R2, to promote activation of downstream pathway components such as Gli proteins. This state is promoted by Shh signal, and can be stabilized by the Smo agonist SAG as well as activating mutations such as SmoM2. IFT-Dynein mediates retrograde transport of Smo (R3) in either an active or inactive state, as Dync2h1 mutant cilia constitutively localize Smo without Shh pathway activation. The mechanisms that deliver Smo to the cilium are not known.
Similar articles
- Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts.
Ocbina PJ, Anderson KV. Ocbina PJ, et al. Dev Dyn. 2008 Aug;237(8):2030-8. doi: 10.1002/dvdy.21551. Dev Dyn. 2008. PMID: 18488998 Free PMC article. - Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation.
Wen X, Lai CK, Evangelista M, Hongo JA, de Sauvage FJ, Scales SJ. Wen X, et al. Mol Cell Biol. 2010 Apr;30(8):1910-22. doi: 10.1128/MCB.01089-09. Epub 2010 Feb 12. Mol Cell Biol. 2010. PMID: 20154143 Free PMC article. - Intraflagellar transport 27 is essential for hedgehog signaling but dispensable for ciliogenesis during hair follicle morphogenesis.
Yang N, Li L, Eguether T, Sundberg JP, Pazour GJ, Chen J. Yang N, et al. Development. 2015 Jun 15;142(12):2194-202. doi: 10.1242/dev.115261. Epub 2015 May 28. Development. 2015. PMID: 26023097 Free PMC article. - Cilia and developmental signaling.
Eggenschwiler JT, Anderson KV. Eggenschwiler JT, et al. Annu Rev Cell Dev Biol. 2007;23:345-73. doi: 10.1146/annurev.cellbio.23.090506.123249. Annu Rev Cell Dev Biol. 2007. PMID: 17506691 Free PMC article. Review. - G-protein-coupled receptors, Hedgehog signaling and primary cilia.
Mukhopadhyay S, Rohatgi R. Mukhopadhyay S, et al. Semin Cell Dev Biol. 2014 Sep;33:63-72. doi: 10.1016/j.semcdb.2014.05.002. Epub 2014 May 17. Semin Cell Dev Biol. 2014. PMID: 24845016 Free PMC article. Review.
Cited by
- Hedgehog signaling activates a positive feedback mechanism involving insulin-like growth factors to induce osteoblast differentiation.
Shi Y, Chen J, Karner CM, Long F. Shi Y, et al. Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):4678-83. doi: 10.1073/pnas.1502301112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825734 Free PMC article. - Potassium Channel KCNH1 Activating Variants Cause Altered Functional and Morphological Ciliogenesis.
Napoli G, Panzironi N, Traversa A, Catalanotto C, Pace V, Petrizzelli F, Giovannetti A, Lazzari S, Cogoni C, Tartaglia M, Carella M, Mazza T, Pizzuti A, Parisi C, Caputo V. Napoli G, et al. Mol Neurobiol. 2022 Aug;59(8):4825-4838. doi: 10.1007/s12035-022-02886-4. Epub 2022 May 31. Mol Neurobiol. 2022. PMID: 35639255 Free PMC article. - Kif3a deficiency reverses the skeletal abnormalities in Pkd1 deficient mice by restoring the balance between osteogenesis and adipogenesis.
Qiu N, Cao L, David V, Quarles LD, Xiao Z. Qiu N, et al. PLoS One. 2010 Dec 2;5(12):e15240. doi: 10.1371/journal.pone.0015240. PLoS One. 2010. PMID: 21151991 Free PMC article. - ARL13B regulates Sonic hedgehog signaling from outside primary cilia.
Gigante ED, Taylor MR, Ivanova AA, Kahn RA, Caspary T. Gigante ED, et al. Elife. 2020 Mar 4;9:e50434. doi: 10.7554/eLife.50434. Elife. 2020. PMID: 32129762 Free PMC article. - Primary cilia in hard tissue development and diseases.
Li S, Zhang H, Sun Y. Li S, et al. Front Med. 2021 Oct;15(5):657-678. doi: 10.1007/s11684-021-0829-6. Epub 2021 Sep 13. Front Med. 2021. PMID: 34515939 Review.
References
- Appel B, Eisen JS. Retinoids run rampant: multiple roles during spinal cord and motor neuron development. Neuron. 2003;40:461–4. - PubMed
- Aza-Blanc P, et al. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–53. - PubMed
- Badano JL, et al. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS044385-03/NS/NINDS NIH HHS/United States
- R01 NS044385-02/NS/NINDS NIH HHS/United States
- R01 NS044385-07/NS/NINDS NIH HHS/United States
- R01 NS044385-01/NS/NINDS NIH HHS/United States
- NS044385/NS/NINDS NIH HHS/United States
- R01 NS044385-06/NS/NINDS NIH HHS/United States
- R01 NS044385-04/NS/NINDS NIH HHS/United States
- R01 NS044385/NS/NINDS NIH HHS/United States
- R01 NS044385-05/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous