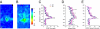Enhanced synaptic connectivity and epilepsy in C1q knockout mice - PubMed (original) (raw)
Enhanced synaptic connectivity and epilepsy in C1q knockout mice
Yunxiang Chu et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Excessive CNS synapses are eliminated during development to establish mature patterns of neuronal connectivity. A complement cascade protein, C1q, is involved in this process. Mice deficient in C1q fail to refine retinogeniculate connections resulting in excessive retinal innervation of lateral geniculate neurons. We hypothesized that C1q knockout (KO) mice would exhibit defects in neocortical synapse elimination resulting in enhanced excitatory synaptic connectivity and epileptiform activity. We recorded spontaneous and evoked field potential activity in neocortical slices and obtained video-EEG recordings from implanted C1q KO and wild-type (WT) mice. We also used laser scanning photostimulation of caged glutamate and whole cell recordings to map excitatory and inhibitory synaptic connectivity. Spontaneous and evoked epileptiform field potentials occurred at multiple sites in neocortical slices from C1q KO, but not WT mice. Laser mapping experiments in C1q KO slices showed that the proportion of glutamate uncaging sites from which excitatory postsynaptic currents (EPSCs) could be evoked ("hotspot ratio") increased significantly in layer IV and layer V, although EPSC amplitudes were unaltered. Density of axonal boutons was significantly increased in layer V pyramidal neurons of C1q KO mice. Implanted KO mice had frequent behavioral seizures consisting of behavioral arrest associated with bihemispheric spikes and slow wave activity lasting from 5 to 30 s. Results indicate that epileptogenesis in C1q KO mice is related to a genetically determined failure to prune excessive excitatory synapses during development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Spontaneous and evoked epileptiform field potentials in cortical layer V of C1q KO brain slices. (A) Representative responses from P14 wild-type (WT) and C1q knockout (KO) animals to layer VI/white matter stimuli. (A1) Threshold stimulation (1×) evokes a short latency biphasic field potential in WT slice. (A2) Superimposed sweeps from a C1q KO slice show two consecutive responses to identical stimuli (0.2 Hz) that evoke either a short latency field potential, or a long duration, polyphasic all-or-none event. (A3) Epileptiform responses were blocked by increasing stimulus intensity to 2× threshold in slice of A2. Arrow marks time of stimulus. (A4) Additional examples of evoked epileptiform field potentials in slice from different C1q KO mouse. Arrows, action potentials (APs). (B) Spontaneous polyphasic epileptiform event with superimposed AP burst (arrow) from a P30 C1q KO brain slice (upper trace). Lower trace shows spontaneous activity from an age-matched, same-sex WT brain slice recorded simultaneously in the same chamber. (C) Spontaneous epileptiform activity from two electrodes spaced ≈1 mm apart in layer V of C1q KO cortical slice. Diagram shows position of the two recording electrodes. (D) Whole cell voltage clamp recording of bursts of spontaneous EPSCs (upper trace) and IPSCs (lower trace) from a C1q KO layer V pyramidal neuron. When the holding potential (V h) is −70 mV, a burst of spontaneous inward polyphasic excitatory currents occurs lasting ≈800 ms. When V h is +20 mV, bursts of spontaneous outward polyphasic inhibitory epileptiform currents are seen in the same neuron. (Scale bar: C, 1 mm.)
Fig. 2.
Excitatory maps to layer V pyramidal neurons in the neocortex of C1q KO mice. (A and B) Average maps of excitatory synaptic connectivity to layer V pyramidal neurons in WT (A) and KO (B) mice. Note that the map in B is expanded significantly (see text). Black triangles, somata of the recorded layer V pyramidal neurons. Pial surface is above the top of map; white matter is on bottom. (C) Region normalized evoked EPSC amplitude, calculated by dividing the total amplitude of evoked events in a stimulus row by total number of spots in that row. (D) Hotspot ratio. (E) Hotspot EPSC amplitude in each row of maps, plotted at different cortical depths relative to the somata (at 0 μm) of layer V pyramidal neurons. (Scale bars: 50 μm). *, P < 0.05; **, P < 0.01; ***, P < 0.005.
Fig. 3.
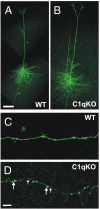
Increased density of axonal boutons in cortical layer V pyramidal neurons of the C1q KO mice. (A and B) Confocal images of biocytin filled layer V pyramidal neurons of C1q KO (A) and WT (B) mice. (C and D) A segment of the axon from the control cell (C) and C1q KO neuron (D). Large and small arrows in D point to examples of large (>1 μm) and small (≤1 μm) boutons, respectively. (Scale bars: A for A and B, 100 μm; D for C and D, 10 μm.)
Fig. 4.
C1q KO mice display spontaneous atypical absence seizures in EEG recordings. (A) Representative EEG trace of a seizure in a P27 C1q KO mouse. Arrows show beginning of behavioral arrest (freezing) at the onset of the seizure and return to normal exploratory behavior after the seizure ends. Horizontal diagram of mouse head shows placement of the four implanted epidural electrodes. (B) EEG recordings for 30 min from P31 WT (B1) and P30 C1q KO (B2) mice, both implanted at age P27, and from the same C1q KO mouse after i.p. injection with 200 mg/kg ethosuximide (B3). Brain waves from the C1q KO mouse show clear periods of seizures. Dotted lines show a blow-up view of a 5-min time trace (B4). (C) Graph depicts a Fourier transform power analysis of EEG frequencies during periods associated with spike activity. C1q KO recordings displayed seizure frequencies of 2–4 Hz, whereas WT recordings and background noise peaked at 7–8 Hz. (D) Total seizure time was significantly reduced with ethosuximide in C1q KO mice. Data were obtained by blind analysis of EEG data using amplitude criteria, accounting for activity in WT. (E) Cumulative probability plot of seizure duration measured in C1q KO mice before and after ethosuximide injection.
Similar articles
- Epileptiform activity and behavioral arrests in mice overexpressing the calcium channel subunit α2δ-1.
Faria LC, Gu F, Parada I, Barres B, Luo ZD, Prince DA. Faria LC, et al. Neurobiol Dis. 2017 Jun;102:70-80. doi: 10.1016/j.nbd.2017.01.009. Epub 2017 Feb 11. Neurobiol Dis. 2017. PMID: 28193459 - Remodeling of dendrites and spines in the C1q knockout model of genetic epilepsy.
Ma Y, Ramachandran A, Ford N, Parada I, Prince DA. Ma Y, et al. Epilepsia. 2013 Jul;54(7):1232-9. doi: 10.1111/epi.12195. Epub 2013 Apr 26. Epilepsia. 2013. PMID: 23621154 Free PMC article. - Increased excitatory connectivity and epileptiform activity in thrombospondin1/2 knockout mice following cortical trauma.
Shu H, Parada I, Delgado A, Prince DA, Gu F. Shu H, et al. Neurobiol Dis. 2024 Oct 1;200:106634. doi: 10.1016/j.nbd.2024.106634. Epub 2024 Aug 7. Neurobiol Dis. 2024. PMID: 39122122 - C1q: the perfect complement for a synaptic feast?
Perry VH, O'Connor V. Perry VH, et al. Nat Rev Neurosci. 2008 Nov;9(11):807-11. doi: 10.1038/nrn2394. Epub 2008 Oct 1. Nat Rev Neurosci. 2008. PMID: 18827829 Review. - Cbln and C1q family proteins: new transneuronal cytokines.
Yuzaki M. Yuzaki M. Cell Mol Life Sci. 2008 Jun;65(11):1698-705. doi: 10.1007/s00018-008-7550-3. Cell Mol Life Sci. 2008. PMID: 18278437 Free PMC article. Review.
Cited by
- Therapeutic Hypothermia Inhibits the Classical Complement Pathway in a Rat Model of Neonatal Hypoxic-Ischemic Encephalopathy.
Shah TA, Pallera HK, Kaszowski CL, Bass WT, Lattanzio FA. Shah TA, et al. Front Neurosci. 2021 Feb 12;15:616734. doi: 10.3389/fnins.2021.616734. eCollection 2021. Front Neurosci. 2021. PMID: 33642979 Free PMC article. - Complement protein C1q modulates neurite outgrowth in vitro and spinal cord axon regeneration in vivo.
Peterson SL, Nguyen HX, Mendez OA, Anderson AJ. Peterson SL, et al. J Neurosci. 2015 Mar 11;35(10):4332-49. doi: 10.1523/JNEUROSCI.4473-12.2015. J Neurosci. 2015. PMID: 25762679 Free PMC article. - Microglia in the developing brain: a potential target with lifetime effects.
Harry GJ, Kraft AD. Harry GJ, et al. Neurotoxicology. 2012 Mar;33(2):191-206. doi: 10.1016/j.neuro.2012.01.012. Epub 2012 Feb 2. Neurotoxicology. 2012. PMID: 22322212 Free PMC article. Review. - Genetic Insights into the Impact of Complement in Alzheimer's Disease.
Torvell M, Carpanini SM, Daskoulidou N, Byrne RAJ, Sims R, Morgan BP. Torvell M, et al. Genes (Basel). 2021 Dec 15;12(12):1990. doi: 10.3390/genes12121990. Genes (Basel). 2021. PMID: 34946939 Free PMC article. Review. - The Complement Regulator Susd4 Influences Nervous-System Function and Neuronal Morphology in Mice.
Zhu H, Meissner LE, Byrnes C, Tuymetova G, Tifft CJ, Proia RL. Zhu H, et al. iScience. 2020 Mar 27;23(3):100957. doi: 10.1016/j.isci.2020.100957. Epub 2020 Feb 28. iScience. 2020. PMID: 32179479 Free PMC article.
References
- O'Leary DD, Stanfield BB. A transient pyramidal tract projection from the visual cortex in the hamster and its removal by selective collateral elimination. Brain Res. 1986;392:87–99. - PubMed
- Killackey HP, Chalupa LM. Ontogenetic change in the distribution of callosal projection neurons in the postcentral gyrus of the fetal rhesus monkey. J Comp Neurol. 1986;244:331–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS12151/NS/NINDS NIH HHS/United States
- R01 NS039579/NS/NINDS NIH HHS/United States
- P01 NS012151/NS/NINDS NIH HHS/United States
- P50 NS012151/NS/NINDS NIH HHS/United States
- K99 NS057940/NS/NINDS NIH HHS/United States
- R01 NS039579-11/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials