Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils - PubMed (original) (raw)
Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils
Mahiuddin Ahmed et al. Nat Struct Mol Biol. 2010 May.
Abstract
The amyloid-beta(1-42) (Abeta42) peptide rapidly aggregates to form oligomers, protofibils and fibrils en route to the deposition of amyloid plaques associated with Alzheimer's disease. We show that low-temperature and low-salt conditions can stabilize disc-shaped oligomers (pentamers) that are substantially more toxic to mouse cortical neurons than protofibrils and fibrils. We find that these neurotoxic oligomers do not have the beta-sheet structure characteristic of fibrils. Rather, the oligomers are composed of loosely aggregated strands whose C termini are protected from solvent exchange and which have a turn conformation, placing Phe19 in contact with Leu34. On the basis of NMR spectroscopy, we show that the structural conversion of Abeta42 oligomers to fibrils involves the association of these loosely aggregated strands into beta-sheets whose individual beta-strands polymerize in a parallel, in-register orientation and are staggered at an intermonomer contact between Gln15 and Gly37.
Figures
Figure 1
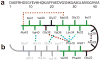
Sequence and structure of the monomer unit in Aβ40 and Aβ42 fibrils. (a) Sequence of Aβ42 that is derived from human APP. (b) Structural constraints in Aβ40 and Aβ42 fibrils. NMR measurements of Aβ40 fibrils have shown that residues 1–10 are unstructured and residues 11–40 adopt a β-turn-β fold ,. Side-chain packing is observed between Phe19 and Ile32/Leu34/Val36, between Gln15 and Val36, and between His13 and Val40 (blue dashed lines). In Aβ42 fibrils, residues 1–17 may be unstructured (in gray) with residues 18–42 forming a β-turn-β fold . Molecular contacts have been reported within the monomer unit of Aβ42 fibrils between Phe19 and Gly38 (red dashed line) and between Met35 and Ala42 (orange dashed line) . In both Aβ40 and Aβ42, the turn conformation is stabilized by hydrophobic interactions (green residues) and by a salt bridge between Asp23 and Lys28 (black dashed line).
Figure 2
Characterization of Aβ42 oligomers, protofibrils and fibrils. (a) TEM of Aβ42 oligomers incubated at 4 °C for 6 h. (b) TEM of Aβ42 protofibrils incubated at 37 °C for 6 h; (c) TEM of Aβ42 fibrils incubated at 37 °C for 12 days. (d) Native gel electrophoresis showing that oligomers contain a single band at ∼20 kDa and that increasing the SDS content can disrupt the oligomeric conformation. (e) Sodium dodecylsulfate (SDS)-polyacrylamide gel electrophoresis of Aβ42 oligomers incubated at 4 °C for 6 h (lane 1) and protofibrils incubated at 37 °C for 6 h (lane 2). Aβ42 was detected using the monoclonal 6E10 anti-Aβ antibody. (f) Cell viability assay of primary cultures of murine cortical neurons treated with Aβ42 oligomers incubated at 4 °C for 6 h or protofibrils incubated at 37 °C for 6 h (n=5). Results represent the mean ± sem, * p < 0.02, ** p <0.001.
Figure 3
Parallel and in-register orientation of β-strands in Aβ42 fibrils. (a) Labeling scheme to test for parallel and in-register orientations of the N- and C-terminal β-strands in Aβ42 fibrils and oligomers using an equimolar mixture of Aβ42-AG1 and Aβ42-AG2 peptides. The red dashed line corresponds to the 4.7 Å distance expected between adjacent Ala21 residues and adjacent Gly37 residues along the fibril axis. (b) Rows through the Ala21 13CO diagonal resonance in DARR NMR spectra of Aβ42 fibrils (red trace) and oligomers (black trace). A distinct Ala21 13CO… Ala21 13Cα cross-peak is observed in the fibril conformation, but not the oligomer conformation. (c) Rows through the Gly37 13CO diagonal resonance in DARR NMR spectra of Aβ42 fibrils (red trace) and oligomers (black trace). A distinct Gly37 13CO… Gly37 13Cα cross-peak is observed in the fibril conformation, but not in the oligomer conformation. Smaller natural abundance (na) cross-peaks are observed in the oligomer samples. Spinning side bands (ssb) due to magic angle spinning are indicated. The data indicate that Aβ42 fibrils have β-strands in a parallel and in-register orientation, but oligomers do not.
Figure 4
Turn structure in Aβ42 fibrils and neurotoxic oligomers. (a) Above, one-dimensional 13C-NMR spectrum showing chemical shift assignments for Phe19, Leu34 and Gly38 in Aβ42 fibrils formed from Aβ42-FLG peptides. Natural abundance (na) cross-peaks are marked with an asterisk. Below, region of the two-dimensional DARR NMR spectrum showing specific 13C…13C molecular contacts between Phe19 and Leu34 (red box) and no contact between Phe19 and Gly38 (blue box) in Aβ42 fibrils. (b) Above, one dimensional 13C-spectrum showing chemical shift assignments for Phe19, Leu34, and Gly38 in Aβ42 oligomers formed from Aβ42-FLG peptides. Below, two-dimensional DARR NMR spectrum showing a specific 13C…13C contact between Phe19 and Leu34 in Aβ42 oligomers. Due to the chemical shift overlap between the Cα of Gly38 and the Cβ of Leu34 in the oligomers, we cannot rule out Phe19-Gly38 contacts in a minor population of Aβ42 peptides. (c) Molecular model of the turn conformation in Aβ42 highlighting the Phe19-Leu34 contact (see also Supplementary Results 1).
Figure 5
β-strands are staggered in Aβ42 fibrils. (a) Above, one dimensional 13C-spectrum showing chemical shift assignments for His13/14, Gln15, Gly37 and Ala42 in Aβ42 fibrils formed from an equimolar mixture of Aβ42-HQA:Aβ42-G37 peptides. Natural abundance 13C assignments are marked with an asterisk. Below, region of the two dimensional DARR NMR spectrum showing specific 13C…13C inter-molecular contacts between Gln15 and Gly37 (red arrow), intra-molecular contacts between Gln15 and His13/14 (orange arrow), and no contact between Gln15 and Ala42, indicating a staggered, domain-swapped architecture. (b) Model of staggering between the N- and C-terminal β-strands at the Gln15-Gly37 contact in Aβ42 fibrils. (c) Above, one dimensional 13C-spectrum showing chemical shift assignments for His13/14, Gln15, Gly37 and Ala42 in Aβ42 oligomers formed from an equimolar mixture of Aβ42-HQA:Aβ42-G37 peptides. Below, two dimensional DARR NMR spectrum showing no molecular contacts between Gln15 and His13/14, Gly37, or Ala42 (red arrow), indicating the absence of a staggered, domain swapped architecture in Aβ42 oligomers. Only a small natural abundance (na) cross-peak is observed (gray arrow).
Figure 6
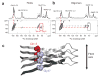
Molecular models of Aβ42 oligomers and fibrils. (a) Schematic of the monomer within the oligomer complex of Aβ42. Solid-state NMR measurements show that Phe19 is in contact with Leu34, while amide exchange measurements suggest there are solvent accessible turns at His13–Gln15, Gly25–Gly29, and Gly37–Gly38. (b) Schematic of the Aβ42 pentamer. The composition of the oligomer is based on SEC and AFM. The orientation of the C-terminus toward the center of the pentamer is based on solvent accessibility. A similar orientation for the hexamer has been proposed by Berstein et al.. (c) Three-dimensional image of single-touch AFM measurements of Aβ42 oligomers. (d) Schematic of the monomer within Aβ42 fibrils. (e) Schematic showing the parallel and in-register packing and staggering of the individual β-strands within Aβ42 fibrils. A single protofilament is shown. Mature fibrils may be formed by the association of 2–3 protofilaments.
Similar articles
- Mechanism of Nucleated Conformational Conversion of Aβ42.
Fu Z, Aucoin D, Davis J, Van Nostrand WE, Smith SO. Fu Z, et al. Biochemistry. 2015 Jul 14;54(27):4197-207. doi: 10.1021/acs.biochem.5b00467. Epub 2015 Jun 26. Biochemistry. 2015. PMID: 26069943 - The Alzheimer's amyloid-β(1-42) peptide forms off-pathway oligomers and fibrils that are distinguished structurally by intermolecular organization.
Tay WM, Huang D, Rosenberry TL, Paravastu AK. Tay WM, et al. J Mol Biol. 2013 Jul 24;425(14):2494-508. doi: 10.1016/j.jmb.2013.04.003. Epub 2013 Apr 11. J Mol Biol. 2013. PMID: 23583777 Free PMC article. - Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.
Kreutzer AG, Nowick JS. Kreutzer AG, et al. Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review. - Resting microglia react to Aβ42 fibrils but do not detect oligomers or oligomer-induced neuronal damage.
Ferrera D, Mazzaro N, Canale C, Gasparini L. Ferrera D, et al. Neurobiol Aging. 2014 Nov;35(11):2444-2457. doi: 10.1016/j.neurobiolaging.2014.05.023. Epub 2014 May 29. Neurobiol Aging. 2014. PMID: 24973120 - Alzheimer's amyloid fibrils: structure and assembly.
Serpell LC. Serpell LC. Biochim Biophys Acta. 2000 Jul 26;1502(1):16-30. doi: 10.1016/s0925-4439(00)00029-6. Biochim Biophys Acta. 2000. PMID: 10899428 Review.
Cited by
- An evaluation of the self-assembly enhancing properties of cell-derived hexameric amyloid-β.
Vadukul DM, Vrancx C, Burguet P, Contino S, Suelves N, Serpell LC, Quinton L, Kienlen-Campard P. Vadukul DM, et al. Sci Rep. 2021 Jun 2;11(1):11570. doi: 10.1038/s41598-021-90680-y. Sci Rep. 2021. PMID: 34078941 Free PMC article. - Glycines from the APP GXXXG/GXXXA Transmembrane Motifs Promote Formation of Pathogenic Aβ Oligomers in Cells.
Decock M, Stanga S, Octave JN, Dewachter I, Smith SO, Constantinescu SN, Kienlen-Campard P. Decock M, et al. Front Aging Neurosci. 2016 May 10;8:107. doi: 10.3389/fnagi.2016.00107. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27242518 Free PMC article. - Dynamics of amyloid β fibrils revealed by solid-state NMR.
Scheidt HA, Morgado I, Rothemund S, Huster D. Scheidt HA, et al. J Biol Chem. 2012 Jan 13;287(3):2017-21. doi: 10.1074/jbc.M111.308619. Epub 2011 Nov 30. J Biol Chem. 2012. PMID: 22130659 Free PMC article. - Varied Probability of Staying Collapsed/Extended at the Conformational Equilibrium of Monomeric Aβ40 and Aβ42.
Song W, Wang Y, Colletier JP, Yang H, Xu Y. Song W, et al. Sci Rep. 2015 Jun 5;5:11024. doi: 10.1038/srep11024. Sci Rep. 2015. PMID: 26046578 Free PMC article. - Islet amyloid polypeptide fibril catalyzes amyloid-β aggregation by promoting fibril nucleation rather than direct axial growth.
Song Z, Tang H, Gatch A, Sun Y, Ding F. Song Z, et al. Int J Biol Macromol. 2024 Nov;279(Pt 1):135137. doi: 10.1016/j.ijbiomac.2024.135137. Epub 2024 Aug 27. Int J Biol Macromol. 2024. PMID: 39208885
References
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
- Kang J, et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. - PubMed
- Jarrett JT, Berger EP, Lansbury PT. The carboxy terminus of the β-amyloid protein is critical for the seeding of amyloid formation - Implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993;32:4693–4697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-AG027317/AG/NIA NIH HHS/United States
- R01 NS035781/NS/NINDS NIH HHS/United States
- DBI-9977553/PHS HHS/United States
- T32 GM008444/GM/NIGMS NIH HHS/United States
- S10 RR13889/RR/NCRR NIH HHS/United States
- R01-NS35781/NS/NINDS NIH HHS/United States
- R01 AG027317/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials