Exposure of hydrophobic surfaces initiates aggregation of diverse ALS-causing superoxide dismutase-1 mutants - PubMed (original) (raw)
Exposure of hydrophobic surfaces initiates aggregation of diverse ALS-causing superoxide dismutase-1 mutants
Christian Münch et al. J Mol Biol. 2010.
Abstract
The copper-zinc superoxide dismutase-1 (SOD1) is a highly structured protein and, a priori, one of the least likely proteins to be involved in a misfolding disease. However, more than 140, mostly missense, mutations in the SOD1 gene cause aggregation of the affected protein in familial forms of amyotrophic lateral sclerosis (ALS). The remarkable diversity of the effects of these mutations on SOD1 properties has suggested that they promote aggregation by a variety of mechanisms. Experimental assessment of surface hydrophobicity using a sensitive fluorescent-based assay, revealed that diverse ALS-causing mutations provoke SOD1 aggregation by increasing their propensity to expose hydrophobic surfaces. These findings could not be anticipated from analysis of the amino acid sequence. Our results uncover the biochemical nature of the misfolded aggregation-prone intermediate and reconcile the seemingly diverse effects of ALS-causing mutations into a unifying mechanism. Furthermore, the method we describe here will be useful for investigating and interfering with aggregation of various proteins and thereby provide insight into the molecular mechanisms underlying many neurodegenerative diseases.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Fig. 1
Diverse properties of fALS-causing SOD1 mutants. (a) Schematic representation of SOD1 structure (PDB entry
1SPD
). Mutants analyzed in this study are highlighted in orange, copper in green and zinc in magenta. Table listing key features of the mutants analyzed in this study. (b) Calculated change of charge (Δcharge) resulting from the mutations. Calculations were performed with PROTEIN CALCULATOR v3.3. (c) Calculated change of hydrophobicity (Δhydrophobicity) resulting from the mutations. Hydrophobicity values were calculated according to, with hydrophobicity values taken form.
Fig. 2
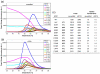
Increased surface hydrophobicity upon thermal denaturation is a generic feature of ALS-causing SOD1 mutants. (a) Sypro Orange fluorescence during thermal unfolding of as-purified SOD1 without or (b) with 20 mM EDTA. Data are means of 3 independent experiments. s.d. are presented in Supplementary Fig. 2. (c) Hydrophobicity values measured in (a and b), as the mean Sypro Orange-derived fluorescence maxima (Fmax) or normalized to the Fmax of SOD1WT, using either as-purified or EDTA-treated proteins.
Fig. 3
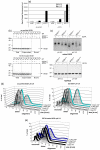
Destabilization of as-purified SOD1A4V by EDTA or low pH exposes hydrophobic surfaces and triggers aggregation. (a) Assessment of surface hydrophobicity by Sypro Orange fluorescence. Means and s.d. of replicate experiments (n = 3) are shown. (b) NuPAGE analysis of SOD1 binding to hydrophobic beads. (c) Electrophoretic mobilities of the SOD1 variants on native PAGE (1.2 μg) or denaturing NuPAGE (0.75 μg). Proteins were treated as indicated 30 min prior electrophoresis. (d, e) Aggregation of SOD1A4V monitored by DLS. d.nm: Diameter in nm.
Fig. 4
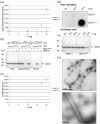
TFE treatment exposes hydrophobic surfaces on as-purified SOD1H46R and provokes fibrillogenesis. (a) Assessment of SOD1H46R surface hydrophobicity by Sypro Orange fluorescence in the presence of increasing TFE concentrations. (b) NuPAGE analysis of SOD1 binding to hydrophobic beads. (c) Aggregation of SOD1H46R monitored by DLS. (d) Aggregation of SOD1H46R revealed by filter retardation assay and NuPAGE analysis of aliquots of the samples used in the filter retardation assay. (e) Electron micrographs of negatively stained SOD1H46R fibrils formed in 20% TFE acquired at magnifications of 7100 (upper panel) and 52000 (lower panel). Means and s.d. of 3 independent experiments are shown in (a and c).
Fig. 5
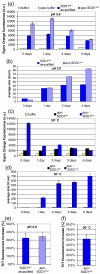
Aggregation of as-purified SOD1 mutants follows exposure of hydrophobic surfaces. (a) Time course of measurements of exposed hydrophobicity monitored by Sypro Orange fluorescence and (b) aggregation of SOD1A4V at acidic pH monitored by DLS. Note that demetallation first provoked a decrease in the size of particles measured, indicating that metal loss provoked monomerization of the protein. (c) Time course of measurements of exposed hydrophobicity and (d) aggregation of apo-SOD1A4V exposed to 50 °C. (e, f) aggregation of SOD1A4V monitored by ThT after 3 days of incubation at acidic pH (e) or 50 °C (f). Data are means and s.d. values of replicate experiments (n = 3).
Fig. 6
Aggregation of as-purified ALS-causing SOD1 mutants is caused by their increased propensity to expose hydrophobic surfaces. (a, b) Correlation between surface hydrophobicity measured by Sypro Orange fluorescence and aggregation measured by DLS (a) and ThT fluorescence (b). Data are means and s.e.m. from 3 independent experiments. Measurements were made 20 minutes after TFE treatment. The high linear correlation coefficient R, denotes the strength of the correlation. Note that the MBR mutants are more susceptible to TFE-induced aggregation than the WTL mutants. (c) Aggregation of SOD1G85R and SOD1G37R at room temperature, revealed by DLS.
Similar articles
- How do ALS-associated mutations in superoxide dismutase 1 promote aggregation of the protein?
Shaw BF, Valentine JS. Shaw BF, et al. Trends Biochem Sci. 2007 Feb;32(2):78-85. doi: 10.1016/j.tibs.2006.12.005. Epub 2007 Jan 5. Trends Biochem Sci. 2007. PMID: 17208444 Review. - Changes in hydrophobicity mainly promotes the aggregation tendency of ALS associated SOD1 mutants.
Tompa DR, Kadhirvel S. Tompa DR, et al. Int J Biol Macromol. 2020 Feb 15;145:904-913. doi: 10.1016/j.ijbiomac.2019.09.181. Epub 2019 Oct 24. Int J Biol Macromol. 2020. PMID: 31669277 - Aberrantly increased hydrophobicity shared by mutants of Cu,Zn-superoxide dismutase in familial amyotrophic lateral sclerosis.
Tiwari A, Xu Z, Hayward LJ. Tiwari A, et al. J Biol Chem. 2005 Aug 19;280(33):29771-9. doi: 10.1074/jbc.M504039200. Epub 2005 Jun 15. J Biol Chem. 2005. PMID: 15958382 - The Disulfide Bond, but Not Zinc or Dimerization, Controls Initiation and Seeded Growth in Amyotrophic Lateral Sclerosis-linked Cu,Zn Superoxide Dismutase (SOD1) Fibrillation.
Chattopadhyay M, Nwadibia E, Strong CD, Gralla EB, Valentine JS, Whitelegge JP. Chattopadhyay M, et al. J Biol Chem. 2015 Dec 18;290(51):30624-36. doi: 10.1074/jbc.M115.666503. Epub 2015 Oct 28. J Biol Chem. 2015. PMID: 26511321 Free PMC article. - Oxidized/misfolded superoxide dismutase-1: the cause of all amyotrophic lateral sclerosis?
Kabashi E, Valdmanis PN, Dion P, Rouleau GA. Kabashi E, et al. Ann Neurol. 2007 Dec;62(6):553-9. doi: 10.1002/ana.21319. Ann Neurol. 2007. PMID: 18074357 Review.
Cited by
- Cellular crowding imposes global constraints on the chemistry and evolution of proteomes.
Levy ED, De S, Teichmann SA. Levy ED, et al. Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20461-6. doi: 10.1073/pnas.1209312109. Epub 2012 Nov 26. Proc Natl Acad Sci U S A. 2012. PMID: 23184996 Free PMC article. - Antigenic and immunogenic properties of recombinant hemagglutinin proteins from H1N1 A/Brisbane/59/07 and B/Florida/04/06 when produced in various protein expression systems.
Santiago FW, Lambert Emo K, Fitzgerald T, Treanor JJ, Topham DJ. Santiago FW, et al. Vaccine. 2012 Jun 29;30(31):4606-16. doi: 10.1016/j.vaccine.2012.05.005. Epub 2012 May 15. Vaccine. 2012. PMID: 22609035 Free PMC article. - Small molecules present in the cerebrospinal fluid metabolome influence superoxide dismutase 1 aggregation.
Cristóvão JS, Leal SS, Cardoso I, Gomes CM. Cristóvão JS, et al. Int J Mol Sci. 2013 Sep 17;14(9):19128-45. doi: 10.3390/ijms140919128. Int J Mol Sci. 2013. PMID: 24048249 Free PMC article. - Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells.
Münch C, O'Brien J, Bertolotti A. Münch C, et al. Proc Natl Acad Sci U S A. 2011 Mar 1;108(9):3548-53. doi: 10.1073/pnas.1017275108. Epub 2011 Feb 14. Proc Natl Acad Sci U S A. 2011. PMID: 21321227 Free PMC article. - ALS-Related Mutant SOD1 Aggregates Interfere with Mitophagy by Sequestering the Autophagy Receptor Optineurin.
Tak YJ, Park JH, Rhim H, Kang S. Tak YJ, et al. Int J Mol Sci. 2020 Oct 13;21(20):7525. doi: 10.3390/ijms21207525. Int J Mol Sci. 2020. PMID: 33065963 Free PMC article.
References
- Bruijn L.I., Miller T.M., Cleveland D.W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004;27:723–749. - PubMed
- Valentine J.S., Doucette P.A., Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu. Rev. Biochem. 2005;74:563–593. - PubMed
- Reaume A.G., Elliott J.L., Hoffman E.K., Kowall N.W., Ferrante R.J., Siwek D.F. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–47. - PubMed
- Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. - PubMed
- Shibata N., Hirano A., Kobayashi M., Siddique T., Deng H.X., Hung W.Y. Intense superoxide dismutase-1 immunoreactivity in intracytoplasmic hyaline inclusions of familial amyotrophic lateral sclerosis with posterior column involvement. J. Neuropathol. Exp. Neurol. 1996;55:481–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous