Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents - PubMed (original) (raw)
. 2010 May;120(5):1506-14.
doi: 10.1172/JCI40096. Epub 2010 Apr 19.
Tohru Minamino, Haruhiro Toko, Sho Okada, Hiroyuki Ikeda, Noritaka Yasuda, Kaoru Tateno, Junji Moriya, Masataka Yokoyama, Aika Nojima, Gou Young Koh, Hiroshi Akazawa, Ichiro Shiojima, C Ronald Kahn, E Dale Abel, Issei Komuro
Affiliations
- PMID: 20407209
- PMCID: PMC2860916
- DOI: 10.1172/JCI40096
Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents
Ippei Shimizu et al. J Clin Invest. 2010 May.
Abstract
Although many animal studies indicate insulin has cardioprotective effects, clinical studies suggest a link between insulin resistance (hyperinsulinemia) and heart failure (HF). Here we have demonstrated that excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. Chronic pressure overload induced hepatic insulin resistance and plasma insulin level elevation. In contrast, cardiac insulin signaling was upregulated by chronic pressure overload because of mechanical stretch-induced activation of cardiomyocyte insulin receptors and upregulation of insulin receptor and Irs1 expression. Chronic pressure overload increased the mismatch between cardiomyocyte size and vascularity, thereby inducing myocardial hypoxia and cardiomyocyte death. Inhibition of hyperinsulinemia substantially improved pressure overload-induced cardiac dysfunction, improving myocardial hypoxia and decreasing cardiomyocyte death. Likewise, the cardiomyocyte-specific reduction of insulin receptor expression prevented cardiac ischemia and hypertrophy and attenuated systolic dysfunction due to pressure overload. Conversely, treatment of type 1 diabetic mice with insulin improved hyperglycemia during pressure overload, but increased myocardial ischemia and cardiomyocyte death, thereby inducing HF. Promoting angiogenesis restored the cardiac dysfunction induced by insulin treatment. We therefore suggest that the use of insulin to control hyperglycemia could be harmful in the setting of pressure overload and that modulation of insulin signaling is crucial for the treatment of HF.
Figures
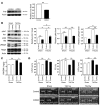
Figure 1. Upregulation of cardiac insulin signals by pressure overload.
(A) Mice were subjected to TAC or sham operation (sham), and heart samples were obtained 2 weeks later. Mice were starved for 6 hours, and insulin or PBS was injected before sacrifice. pIrs1 and pAkt levels in the heart were examined by Western blot analysis. The graphs indicate relative expression levels of pIrs1 and pAkt. n = 3. TAC2w, 2 weeks after TAC. (B) Mice were subjected to TAC or sham operation and were sacrificed 2 weeks later. Components of the insulin signaling pathway in the heart were examined by Western blot analysis. The graphs indicate relative expression levels of these signaling molecules. n = 3. Data are shown as mean ± SEM. *P < 0.05; **P < 0.01.
Figure 2. Depletion of plasma insulin attenuates systolic dysfunction induced by pressure overload.
(A) STZ- or vehicle-treated mice were subjected to TAC or sham operation. The heart weight/body weight (HW/BW) ratio was measured 2 weeks after operation. In the insulin-treated group, daily i.p. injection of insulin (0.1 IU/g/d) was performed from 9 weeks (2 weeks after STZ treatment) to 13 weeks of age (2 weeks after TAC). n = 22–24. (B) Cardiac hypertrophy and systolic function of the animals prepared for A were estimated by echocardiography at 1 week (IVST) or 2 weeks (FS and LVDs) after operation. Photographs show representative results of echocardiography (M-mode). n = 6–10. Data are shown as mean ± SEM. *P < 0.05; **P < 0.01. IVST, intraventricular septal thickness; FS, fractional shortening.
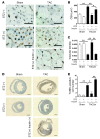
Figure 3. Reduction of plasma insulin inhibits cardiac hypoxia due to pressure overload.
(A) Animals were prepared as described for Figure 2A. Immunohistochemistry using antibodies against platelet and endothelial cell adhesion molecule (dark brown) and dystrophin (light brown) was performed at 2 weeks after operation. Scale bars: 20 μm. (B and C) CSA of cardiomyocytes (B) and relative vascular density (C) were estimated as described in Methods. n = 4–5. (D) Cardiac ischemia (brown) in mice prepared as described for Figure 2A was estimated with a Hypoxyprobe-1. Scale bars: 1 mm. (E) Number of TUNEL-positive cells per 1 × 104 cardiomyocytes. n = 4–6. Data are shown as mean ± SEM. *P < 0.05; **P < 0.01.
Figure 4. Cardiomyocyte-specific reduction of Insr expression attenuates systolic dysfunction due to pressure overload.
(A) Western blot analysis of Insr expression in the hearts of CIRKO mice (_Insrflox/+_Cre+) and their littermate controls (control). Graphs indicate relative expression levels of Insr. n = 3. (B) CIRKO mice (_Insrflox/+_Cre+) or littermate controls were subjected to TAC or sham operation, and components of the insulin signaling pathway in the heart were examined by Western blot analysis at 2 weeks after operation. Graphs indicate relative expression levels of these signaling molecules. n = 3. (C) The heart weight/body weight ratio of animals prepared as described in A was measured at 2 weeks after operation. n = 7–9. (D) Cardiac hypertrophy and systolic function of animals prepared as described in A were assessed by echocardiography at 1 week (IVST) or 2 weeks (FS and LVDs) after operation. Photographs show representative results of echocardiography (M-mode). n = 8–13. Data are shown as mean ± SEM. *P < 0.05; **P < 0.01.
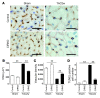
Figure 5. Cardiomyocyte-specific reduction of Insr expression attenuates cardiac hypoxia due to pressure overload.
(A) CIRKO mice (_Insrflox/+_Cre+) or littermate controls were subjected to TAC or sham operation. Immunohistochemistry using antibodies against platelet and endothelial cell adhesion molecules (dark brown) and dystrophin (light brown) was performed at 2 weeks after operation. Scale bars: 20 μm. (B and C) CSA of cardiomyocytes (B) and relative vascular density (C) were estimated as described in Methods. n = 4–5. (D) Number of TUNEL-positive cells per 1 × 104 cardiomyocytes. n = 4–5. Data are shown as mean ± SEM. *P < 0.05; **P < 0.01.
Figure 6. Reduced activation of Akt attenuates systolic dysfunction due to pressure overload.
(A) _Akt1_-deficient (Akt1+/–) mice and WT littermates were subjected to TAC or sham operation. Cardiac hypertrophy and systolic function were assessed by echocardiography at 2 weeks after operation. n = 4–6. (B) Immunohistochemistry using antibodies against platelet and endothelial cell adhesion molecules (dark brown) and dystrophin (light brown) was performed at 2 weeks after operation. Scale bars: 20 μm. (C) CSA of cardiomyocytes and relative vascular density were estimated as described in Methods. n = 3. (D) pAkt and Akt levels in the heart at 2 weeks after operation were examined by Western blot analysis. Graphs indicate relative expression levels of pAkt and Akt. n = 3. Data are shown as mean ± SEM. *P < 0.05; **P < 0.01.
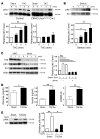
Figure 7. Mechanism of enhanced insulin signaling in the heart during pressure overload.
(A) CIRKO mice (_Insrflox/flox_Cre+) or littermate controls were subjected to TAC or sham operation, and heart samples were obtained at the indicated times. pIrs1 levels were examined by Western blot analysis. The graphs indicate relative expression levels of pIrs1. n = 3. (B) Cardiomyocytes were subjected to mechanical stretch and pIrs1 levels were examined by Western blot analysis. n = 3. (C) siRNA targeting Insr (siInsr) or negative control RNA (siNC) was introduced into cardiomyocytes, after which the cells were subjected to mechanical stretch. pIrs1 levels were examined by Western blot analysis. n = 3. (D) Plasma glucose and insulin levels were examined at 2 weeks after TAC. n = 7–8. (E) Insulin-induced phosphorylation of Akt (pAkt) in the liver was examined after TAC or sham operation. n = 3. Data are shown as mean ± SEM. *P < 0.05; **P < 0.01.
Similar articles
- Cardiomyocyte-specific ablation of CD36 accelerates the progression from compensated cardiac hypertrophy to heart failure.
Sung MM, Byrne NJ, Kim TT, Levasseur J, Masson G, Boisvenue JJ, Febbraio M, Dyck JR. Sung MM, et al. Am J Physiol Heart Circ Physiol. 2017 Mar 1;312(3):H552-H560. doi: 10.1152/ajpheart.00626.2016. Epub 2017 Jan 6. Am J Physiol Heart Circ Physiol. 2017. PMID: 28062415 - Mixed lineage kinase-3 prevents cardiac dysfunction and structural remodeling with pressure overload.
Calamaras TD, Baumgartner RA, Aronovitz MJ, McLaughlin AL, Tam K, Richards DA, Cooper CW, Li N, Baur WE, Qiao X, Wang GR, Davis RJ, Kapur NK, Karas RH, Blanton RM. Calamaras TD, et al. Am J Physiol Heart Circ Physiol. 2019 Jan 1;316(1):H145-H159. doi: 10.1152/ajpheart.00029.2018. Epub 2018 Oct 26. Am J Physiol Heart Circ Physiol. 2019. PMID: 30362822 Free PMC article. - Cardiomyocyte-restricted inhibition of G protein-coupled receptor kinase-3 attenuates cardiac dysfunction after chronic pressure overload.
von Lueder TG, Gravning J, How OJ, Vinge LE, Ahmed MS, Krobert KA, Levy FO, Larsen TS, Smiseth OA, Aasum E, Attramadal H. von Lueder TG, et al. Am J Physiol Heart Circ Physiol. 2012 Jul;303(1):H66-74. doi: 10.1152/ajpheart.00724.2011. Epub 2012 Apr 27. Am J Physiol Heart Circ Physiol. 2012. PMID: 22542621 - Steroid receptor coactivator-2 (SRC-2) coordinates cardiomyocyte paracrine signaling to promote pressure overload-induced angiogenesis.
Suh JH, Lai L, Nam D, Kim J, Jo J, Taffet GE, Kim E, Kaelber JT, Lee HK, Entman ML, Cooke JP, Reineke EL. Suh JH, et al. J Biol Chem. 2017 Dec 29;292(52):21643-21652. doi: 10.1074/jbc.M117.804740. Epub 2017 Nov 10. J Biol Chem. 2017. PMID: 29127200 Free PMC article. - Young Investigator Award: Compound A, a Ginger Extract, Significantly Reduces Pressure Overload-induced Systolic Heart Failure in Mice.
Kawase Y, Shimizu K, Funamoto M, Sunagawa Y, Katanasaka Y, Miyazaki Y, Shimizu S, Hasegawa K, Morimoto T. Kawase Y, et al. Eur Cardiol. 2021 Dec 14;16:e57. doi: 10.15420/ecr.2021.16.PO1. eCollection 2021 Feb. Eur Cardiol. 2021. PMID: 35106073 Free PMC article. Review. No abstract available.
Cited by
- Insulin signaling in the heart.
Abel ED. Abel ED. Am J Physiol Endocrinol Metab. 2021 Jul 1;321(1):E130-E145. doi: 10.1152/ajpendo.00158.2021. Epub 2021 May 31. Am J Physiol Endocrinol Metab. 2021. PMID: 34056923 Free PMC article. Review. - Insulin and Insulin-Like Growth Factor 1 Signaling Preserves Sarcomere Integrity in the Adult Heart.
Riehle C, Weatherford ET, McCarty NS, Seei A, Jaishy BP, Manivel R, Galuppo P, Allamargot C, Hameed T, Boudreau RL, Bauersachs J, Weiss RM, Abel ED. Riehle C, et al. Mol Cell Biol. 2022 Oct 20;42(10):e0016322. doi: 10.1128/mcb.00163-22. Epub 2022 Sep 20. Mol Cell Biol. 2022. PMID: 36125265 Free PMC article. - Reconstituted HDL (Milano) Treatment Efficaciously Reverses Heart Failure with Preserved Ejection Fraction in Mice.
Mishra M, Muthuramu I, Aboumsallem JP, Kempen H, De Geest B. Mishra M, et al. Int J Mol Sci. 2018 Oct 30;19(11):3399. doi: 10.3390/ijms19113399. Int J Mol Sci. 2018. PMID: 30380754 Free PMC article. - Molecular mechanisms of diabetic cardiomyopathy.
Bugger H, Abel ED. Bugger H, et al. Diabetologia. 2014 Apr;57(4):660-71. doi: 10.1007/s00125-014-3171-6. Epub 2014 Jan 30. Diabetologia. 2014. PMID: 24477973 Free PMC article. Review. - Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy.
Kitada K, Nakano D, Ohsaki H, Hitomi H, Minamino T, Yatabe J, Felder RA, Mori H, Masaki T, Kobori H, Nishiyama A. Kitada K, et al. J Diabetes Complications. 2014 Sep-Oct;28(5):604-11. doi: 10.1016/j.jdiacomp.2014.05.010. Epub 2014 Jun 4. J Diabetes Complications. 2014. PMID: 24996978 Free PMC article.
References
- Adams TD, Yanowitz FG, Fisher AG, Ridges JD, Lovell K, Pryor TA. Noninvasive evaluation of exercise training in college-age men. Circulation. 1981;64(5):958–965. - PubMed
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous