Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells - PubMed (original) (raw)
Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells
Yohsuke Harada et al. J Exp Med. 2010.
Abstract
The transcription factor Foxp3 is essential for optimal regulatory T (T reg) cell development and function. Here, we show that CD4(+) T cells from Cbl-b RING finger mutant knockin or Cbl-b-deficient mice show impaired TGF-beta-induced Foxp3 expression. These T cells display augmented Foxo3a phosphorylation, but normal TGF-beta signaling. Expression of Foxo3a rescues Foxp3 expression in Cbl-b-deficient T cells, and Foxo3a deficiency results in defective TGF-beta-driven Foxp3 induction. A Foxo3a-binding motif is present in a proximal region of the Foxp3 promoter, and is required for Foxo3a association. Foxo1 exerts similar effects as Foxo3a on Foxp3 expression. This study reveals that Foxo factors promote transcription of the Foxp3 gene in induced T reg cells, and thus provides new mechanistic insight into Foxo-mediated T cell regulation.
Figures
Figure 1.
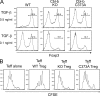
The requirement of Cbl-b E3 ligase activity in the regulation of Foxp3+ iT reg cells. (A) Naive CD4+CD62L+CD25− T cells from wild-type (WT), Cbl-b–deficient (KO), and Cbl-b C373A knockin mice were stimulated with anti-CD3 and anti-CD28 in the presence of indicated concentrations of TGF-β. Development of Foxp3+ iT reg cells was assessed by FACS analysis after 5 d. The percentages of Foxp3-expressing cells are shown. Data are representative of five independent experiments. (B) The cells in A were co-cultured with CFSE-labeled CD4+CD25− T eff cells at a 1:1 ratio in the presence of irradiated T cell–depleted splenocytes and anti-CD3. CFSE dilution was assessed 4 d later by FACS analysis. Data are representative of at least three independent experiments.
Figure 2.
Cbl-b deficiency attenuates Foxp3+ iT reg cell differentiation in vivo. Naive OTII T cells (Vβ 5.1/5.2+) from WT and Cbl-b KO mice (CD45.2) were adoptively transferred to B6 SJL (CD45.1) mice, and the recipients were immunized with 10 µg of OVA323-339 peptide (OVAp). 5 d after immunization, Foxp3 expression in CD45.2+TCR Vβ 5.1/5.2+ T cells were measured. ABI, axillary, branchial, and inguinal lymph nodes. (A) Representative FACS plots showing the percentage of Foxp3-expressing 5.1/5.2+ donor OTII T cells. Data are representative of three independent experiments. (B) Statistical representation of Foxp3 induction in donor OTII T cells. Each dot represents an individual mouse. Small horizontal lines indicate the mean, and error bars indicate standard deviations. **, P < 0.01; ***, P < 0.001. Data are from three independent experiments.
Figure 3.
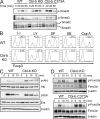
PI3K–Akt–Foxo3a activation in Cbl-b–mediated regulation of Foxp3+ iT reg cells. (A) Phosphorylation of Smad proteins in naive CD4+ T cells from WT, Cbl-b KO, and Cbl-b C373A mice. Naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 in the presence of TGF-β for indicated time periods. Whole-cell lysates were immunoblotted with anti–phospho-(p)-Smad2, or anti–p-Smad3, and reprobed with anti-Smad2/3. Smad2, 60 kD; Smad3, 52 kD. Data are representative of three independent experiments. (B) Naive CD4+ T cells from WT and Cbl-b KO mice were stimulated with anti-CD3 and anti-CD28 together with TGF-β in the absence or presence of the PI3K inhibitor LY294002 (LY), the JNK inhibitor SP600125 (SP), the p38 inhibitor SB203580 (SB), or the calcineurin inhibitor cyclosporine A (Csp A). Foxp3 expression was assessed by FACS analysis after 5 d. The percentages of Foxp3-expressing cells are shown. Data are representative of three independent experiments. (C) Naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 for the indicated time periods. Whole-cell lysates were immunoblotted with anti–p-Akt, anti-Akt, anti–p-Erk, anti-Erk2, and anti–β-actin. Akt, 56 kD; Erk, 42/44 kD; β-actin, 45 kD. Data are representative of at least three independent experiments. (D) Regulation of Foxo3a phosphorylation by Cbl-b E3 ligase. Naive CD4+ T cells from WT and Cbl-b KO mice (top) or WT and Cbl-b C373A mice (bottom) were stimulated with anti-CD3 and anti-CD28 for indicated time periods. Whole-cell lysates were immunoblotted with anti–p-Foxo3a, anti-Foxo3a, and anti–β-actin. Foxo3a, 95 kD; β-actin, 45 kD. Data are representative of at least three independent experiments.
Figure 4.
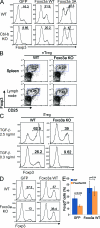
Foxo3a regulates Foxp3+ iT reg cell differentiation. (A) Naive CD4+ T cells from WT and Cbl-b KO mice were stimulated with anti-CD3 and anti-CD28 for 2 d and retrovirally transduced with control-IRES-GFP (GFP), Foxo3a WT-IRES-GFP (Foxo3a WT), or Foxo3a 3A-IRES-GFP (Foxo3a 3A). After infection, TGF-β was added and Foxp3 expression was assessed 3 d later by FACS analysis. The percentages of Foxp3-expressing cells in gated GFP-positive cells are shown. Data are representative of four independent experiments. (B) nT reg cells in the spleen and lymph nodes of Foxo3a KO mice. The spleen and the lymph node cells from WT and Foxo3a KO mice were stained with anti-CD4, anti-CD25, and anti-Foxp3. The percentages of Foxp3-expressing cells in CD4 T cells are shown. Data are representative of three independent experiments. (C) Naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 in the presence of indicated concentrations of TGF-β for 3 d. Foxp3 expression was assessed by FACS analysis. The percentages of Foxp3-expressing cells in gated CD4 T cells are shown. Data are representative of five independent experiments. (D) Naive CD4 T cells from WT and Foxo3a KO mice were stimulated with anti-CD3 and anti-CD28 for 2 d and retrovirally transduced with control-IRES-GFP (GFP) or Foxo3a WT-IRES-GFP (Foxo3a WT). 1 d after infection, TGF-β was added, and Foxp3 expression was assessed 3 d later by FACS analysis. A representative of three repeated experiments. (E) The percentages of Foxp3-expressing cells in GFP-positive cells as shown in D are calculated and shown as mean ± SD of three independent experiments. Statistical significance of the data were evaluated by unpaired two-tailed Student’s t test.
Figure 5.
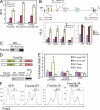
Foxo3a directly binds to the Foxp3 promoter. (A) 293T cells were transfected with luciferase reporter plasmids containing the Foxp3 promoter, the promoter and the Foxp3 enhancer 1 (E1), or the promoter and the Foxp3 enhancer 2 (E2), together with an empty, Foxo3a WT, or Foxo3a 3A expression vector. Bars show the mean relative luciferase unit (RLU) ± SD as arbitrary light units of three independent experiments. (B, top) Schematic structure of the Foxp3 promoter region and mutated sequence of the Foxp3 promoter. (bottom) Jurkat cells were transfected with luciferase reporter plasmids containing the Foxp3 promoter and the Foxp3 enhancer 1 or the Foxp3 promoter with Foxo3a binding site mutated and the Foxp3 enhancer 1 (WT, Mut1, Mut2, and Mut3) together with an empty, Foxo3a WT, Foxo3a 3A, Runx1, or Smad3 expression vectors. 24 h after transfection, these cells were stimulated with anti-CD3 and anti-CD28 for 8 h. Bars show the mean RLU ± SD as arbitrary light units of three independent experiments. (C) Pull-down assay of Foxo3a binding to a WT or mutated (Mut3) sequence of the Foxp3 promoter. Cell lysates from naive CD4 T cells stimulated with anti-CD3, anti-CD28, and TGF-β were mixed with biotinylated DNA probes. The labeled DNA probes were precipitated with streptavidin-agarose beads, and the precipitates were subjected to SDS-PAGE, followed by immunoblotting with anti-Foxo3a. Input represents 5% of the total amount used for precipitation. Foxo3a, 95 kD. Data are representative of three independent experiments. (D, top) Schematic structure of Foxo3a WT and H212R mutant. (bottom) 293T cells were transfected with myc-tagged Foxo3a WT or H212R mutant. The cell lysates were mixed with biotinylated DNA probes encoding WT or Mut3 Foxp3 promoters and pull-down assay was performed as in C. Foxo3a was detected by immunoblotting with anti-myc to detect myc-tagged Foxo3a proteins. Foxo3a, 95 kD. Data are representative of three independent experiments. (E) Chromatin immunoprecipitation analysis of Foxo3a binding to the Foxp3 promoter region. Naive CD4 T cells were unstimulated or stimulated with anti-CD3 and anti-CD28 together with TGF-β. Cell lysates were immunoprecipitated with anti-Foxo3a or control IgG. Immunoprecipitates from WT and Foxo3a KO T cells were analyzed by quantitative real-time PCR, using primers corresponding to Foxp3 promoter, its enhancer 1, and a nonspecific Actin promoter as a control. The results were presented as fold of template enrichment in immunoprecipitates of anti-Foxo3a relative to those of control IgG (mean and SD of three independent experiments). (F) Naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 for 2 d and retrovirally transduced with control-IRES-GFP (GFP), Foxo3a WT-IRES-GFP (Foxo3a WT), Foxo3a 3A-IRES-GFP (Foxo3a 3A), or Foxo3a 3A H212R-IRES-GFP (Foxo3a 3A H212R). After infection, TGF-β was added and Foxp3 expression was assessed 3 d later by FACS analysis. The percentages of Foxp3-expressing cells in GFP positive cells are shown. Data are representative of at least three independent experiments.
Figure 6.
Foxo1 regulates Foxp3 expression. (A and B) Naive CD4+ T cells from WT and Cbl-b KO mice (A) or WT and Cbl-b C373A mice (B) were stimulated with anti-CD3 and anti-CD28 for indicated time periods. Whole-cell lysates were immunoblotted with anti–p-Foxo1, anti-Foxo1 (75 kD), and anti–β-actin (45 kD). Data are representative of three independent experiments. (C) Naive CD4+ T cells were stimulated with anti-CD3 and anti-CD28 for 2 d and transduced with retroviruses encoding Foxo1-specific shRNAs (shRNA1 or shRNA2) or with empty LMP vector (control). After 24 h, TGF-β was added and Foxp3 expression was assessed 3 d later by FACS analysis. Cells were treated with puromycin for last 48 h. The percentages of Foxp3-expressing cells in GFP-positive cells are shown. Data are representative of three independent experiments. (D) Cell lysates in (C) were immunoblotted with anti-Foxo1 (75 kD), anti-Foxo3a (95 kD), and anti-β-actin (45 kD). Data are representative of three independent experiments. (E) Naive CD4 T cells were stimulated with anti-CD3 and anti-CD28 for 2 d and retrovirally transduced with control-IRES-GFP (GFP), Foxo1 WT-IRES-GFP (Foxo1 WT), or Foxo3a WT-IRES-GFP (Foxo3a WT). After infection, TGF-β was added and Foxp3 expression was assessed 3 d later by FACS analysis. The percentages of Foxp3-expressing cells in GFP-positive cells are shown. Data are representative of four independent experiments. (F) Pull-down assay of Foxo1 binding to WT or mutated (Mut3) sequence of the Foxp3 promoter. DNA pull-down assay was performed using biotinylated DNA probes. The precipitates were subjected to SDS-PAGE, followed by immunoblotting with anti-Foxo1 (75 kD). Data are representative of at least three independent experiments.
Similar articles
- T cell activation threshold regulated by E3 ubiquitin ligase Cbl-b determines fate of inducible regulatory T cells.
Qiao G, Zhao Y, Li Z, Tang PQ, Langdon WY, Yang T, Zhang J. Qiao G, et al. J Immunol. 2013 Jul 15;191(2):632-9. doi: 10.4049/jimmunol.1202068. Epub 2013 Jun 7. J Immunol. 2013. PMID: 23749633 Free PMC article. - Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo.
Wohlfert EA, Gorelik L, Mittler R, Flavell RA, Clark RB. Wohlfert EA, et al. J Immunol. 2006 Feb 1;176(3):1316-20. doi: 10.4049/jimmunol.176.3.1316. J Immunol. 2006. PMID: 16424156 - Novel Foxo1-dependent transcriptional programs control T(reg) cell function.
Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, Chan P, Ma Q, Mo Y, Meijer D, Zhao K, Rudensky AY, Atwal G, Zhang MQ, Li MO. Ouyang W, et al. Nature. 2012 Nov 22;491(7425):554-9. doi: 10.1038/nature11581. Epub 2012 Nov 7. Nature. 2012. PMID: 23135404 Free PMC article. - Cbl-b and itch: key regulators of peripheral T-cell tolerance.
Venuprasad K. Venuprasad K. Cancer Res. 2010 Apr 15;70(8):3009-12. doi: 10.1158/0008-5472.CAN-09-4076. Cancer Res. 2010. PMID: 20395198 Free PMC article. Review. - Ubiquitination signals critical to regulatory T cell development and function.
Chen Z, Luo X, Lu Y, Zhu T, Wang J, Tsun A, Li B. Chen Z, et al. Int Immunopharmacol. 2013 Jul;16(3):348-52. doi: 10.1016/j.intimp.2013.01.023. Epub 2013 Feb 14. Int Immunopharmacol. 2013. PMID: 23415874 Review.
Cited by
- Protein kinase C-θ inhibits inducible regulatory T cell differentiation via an AKT-Foxo1/3a-dependent pathway.
Ma J, Ding Y, Fang X, Wang R, Sun Z. Ma J, et al. J Immunol. 2012 Jun 1;188(11):5337-47. doi: 10.4049/jimmunol.1102979. Epub 2012 Apr 25. J Immunol. 2012. PMID: 22539794 Free PMC article. - mTOR and metabolic pathways in T cell quiescence and functional activation.
Yang K, Chi H. Yang K, et al. Semin Immunol. 2012 Dec;24(6):421-8. doi: 10.1016/j.smim.2012.12.004. Epub 2013 Feb 1. Semin Immunol. 2012. PMID: 23375549 Free PMC article. Review. - Cbl-b: Roles in T Cell Tolerance, Proallergic T Cell Development, and Cancer Immunity.
Zhang J, Liu Q, Langdon WY. Zhang J, et al. Inflamm Cell Signal. 2014;1(4):e146. doi: 10.14800/ics.146. Inflamm Cell Signal. 2014. PMID: 26082933 Free PMC article. - Regulation of immune responses by E3 ubiquitin ligase Cbl-b.
Tang R, Langdon WY, Zhang J. Tang R, et al. Cell Immunol. 2019 Jun;340:103878. doi: 10.1016/j.cellimm.2018.11.002. Epub 2018 Nov 7. Cell Immunol. 2019. PMID: 30442330 Free PMC article. Review. - TGF-β1 Drives Inflammatory Th Cell But Not Treg Cell Compartment Upon Allergen Exposure.
Musiol S, Alessandrini F, Jakwerth CA, Chaker AM, Schneider E, Guerth F, Schnautz B, Grosch J, Ghiordanescu I, Ullmann JT, Kau J, Plaschke M, Haak S, Buch T, Schmidt-Weber CB, Zissler UM. Musiol S, et al. Front Immunol. 2022 Jan 7;12:763243. doi: 10.3389/fimmu.2021.763243. eCollection 2021. Front Immunol. 2022. PMID: 35069535 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous