The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner - PubMed (original) (raw)
. 2010 May 18;107(20):9440-5.
doi: 10.1073/pnas.0914801107. Epub 2010 May 3.
Adrian Butcher, Phillip McWilliams, Julie-Myrtille Bourgognon, Robert Pawlak, Kok Choi Kong, Andrew Bottrill, Sharad Mistry, Jürgen Wess, Elizabeth M Rosethorne, Steven J Charlton, Andrew B Tobin
Affiliations
- PMID: 20439723
- PMCID: PMC2889095
- DOI: 10.1073/pnas.0914801107
The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner
Benoit Poulin et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Degeneration of the cholinergic system is considered to be the underlying pathology that results in the cognitive deficit in Alzheimer's disease. This pathology is thought to be linked to a loss of signaling through the cholinergic M(1)-muscarinic receptor subtype. However, recent studies have cast doubt on whether this is the primary receptor mediating cholinergic-hippocampal learning and memory. The current study offers an alternative mechanism involving the M(3)-muscarinic receptor that is expressed in numerous brain regions including the hippocampus. We demonstrate here that M(3)-muscarinic receptor knockout mice show a deficit in fear conditioning learning and memory. The mechanism used by the M(3)-muscarinic receptor in this process involves receptor phosphorylation because a knockin mouse strain expressing a phosphorylation-deficient receptor mutant also shows a deficit in fear conditioning. Consistent with a role for receptor phosphorylation, we demonstrate that the M(3)-muscarinic receptor is phosphorylated in the hippocampus following agonist treatment and following fear conditioning training. Importantly, the phosphorylation-deficient M(3)-muscarinic receptor was coupled normally to G(q/11)-signaling but was uncoupled from phosphorylation-dependent processes such as receptor internalization and arrestin recruitment. It can, therefore, be concluded that M(3)-muscarinic receptor-dependent learning and memory depends, at least in part, on receptor phosphorylation/arrestin signaling. This study opens the potential for biased M(3)-muscarinic receptor ligands that direct phosphorylation/arrestin-dependent (non-G protein) signaling as being beneficial in cognitive disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
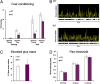
Fig. 1.
Fear conditioning response in wild-type and M3R-KO mice. Wild-type (WT) or M3-muscarinic receptor knockout mice (KO) were subjected to fear conditioning training. 24 h after training mice were analyzed for contextual fear conditioning and 24 h after this the animals were analyzed for cued fear conditioning. (A) Cumulative fear conditioning responses in WT (n = 10) and KO (n = 10) mice. (B) Example of a single movement trace from WT and a KO mouse undergoing a test for contextual fear conditioning. Freezing is defined when movement amplitude falls below the white line. Length and frequency of freezing is also represented by the yellow bars on the bar graph. (C) Measure for anxiety was conducted on WT (n = 10) and KO (n = 10) mice using the elevated plus maze. (D) Pain thresholds determined in WT (n = 10) and KO (n = 10) mice. Data represents the means ± SE. *, P < 0.01 (t test).
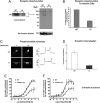
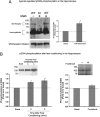
Fig. 2.
Characterization of the phosphorylation-deficient M3-muscarinic receptor. (A) Phosphorylation of the M3-muscarinic receptor was determined in CG neurons derived from wild-type (WT) or M3R-KI (KI) mice metabolically labeled with [32P]orthophosphate. CG neurons were stimulated with or without methacholine (Meth, 100 μM) for 5 min before solubilization and immunoprecipitation of the M3-muscarinic receptor. Shown is an example autoradiograph (showing long and short exposures) and the Western blot loading control (M3-Receptor Western). (B) Quantification of the receptor phosphorylation data shown in A. The data represents the mean ± SE from four independent experiments. (C) Coupling of the M3-muscarinic receptor to the phosphoinositide pathway was determined in CG neurons derived from wild-type (WT) or M3R-KI (KI) mice transfected with the phosphoinositide biosensor (eGFP-PHPLCδ1). CG neurons were stimulated with methacholine (0.1 mM; downward arrow) for the indicated time (the bar represents 30 s). Agonist was then removed (upward arrow). The translocation of the biosensor from the plasma membrane to the cytoplasm was monitored on an inverted epifluorescence microscope. Shown are images of a single neuron before and during stimulation with methacholine. On the left is a representative time course of fluorescence change in the cytoplasm expressed as a self ratio (F/F0). (D) Internalization of M3-muscarinic receptors expressed in CG neurons derived from wild-type (WT) and M3R-KI (KI) mice determined by stimulating cultures at 37 °C in the absence or presence of methacholine (0.1 mM) for 30 min. Shown is the mean percentage receptor internalization ± SE (n = 5). (E and F) β-Arrestin recruitment to the M3-muscarinic receptor determined using PathHunter CHO-K1 cells expressing either the wild-type human M3-muscarinic receptor (WT) or the mutated human M3-muscarinic receptor lacking phospho-acceptor sites (H-M3phos-neg) (18). Shown are concentration response curves to the full muscarinic receptor agonists methacholine and acetylcholine. The data are the means ± SE of three independent experiments.
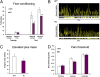
Fig. 3.
Fear conditioning response in wild-type and M3R-KI mice. Wild-type (WT) or M3R-KI (KI) mice were subjected to fear conditioning training. Twenty-four hours after training, mice were analyzed for contextual fear conditioning, and 24 h later the animals were analyzed for cued fear conditioning. (A) Cumulative fear conditioning responses in WT (n = 10) and M3R-KI (n = 10) mice. (B) Example of a single movement trace from a WT and a M3R-KI (KI) mouse undergoing a test for contextual fear conditioning. Freezing is defined when movement amplitude falls below the white line. Length and frequency of freezing are also represented by the yellow bars on the bar graph. (C) Measure for anxiety was conducted on WT (n = 10) and M3R-KI (n = 10) mice using the elevated plus maze. (D) Pain thresholds were determined in WT (n = 10) and M3R-KI (n = 10) mice. Data represents means ± SE. *, P < 0.05 (t test).
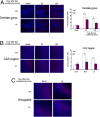
Fig. 4.
c-Fos induction in the hippocampus and amygdala following fear conditioning depends on M3-muscarinic receptor phosphorylation. Wild-type (WT) or M3R-KI (KI) mice were subjected to fear conditioning training, and the animals were killed by cardiac perfusion of fixative solution 30 or 120 min after training. The brain was then dissected and sectioned before staining for c-Fos expression. Basal conditions represent animals that had not undergone fear conditioning training. (A) Immunofluorescent staining for c-Fos expression in the dentate gyrus of the hippocampus. (B) Immunofluorescent staining for c-Fos expression in the CA3 region of the hippocampus. (C) Immunofluorescent staining for c-Fos expression in the amygdala. Graphs represent quantification of the immunofluorescent staining showing means ± SE (n = 4). *, P < 0.05 (t test). c-Fos appears as the red label and nuclei are stained blue using DAPI.
Fig. 5.
Phosphorylation of serine384 on the M3-muscarinic receptor in the hippocampus. (A) Hippocampi dissected from wild-type (WT) or M3R-KI (KI) mice were exposed to vehicle or methacholine (Meth, 1 mM) for 10 min. Membranes were then prepared and solubilized. The M3-muscarinic receptor was then immunoprecipitated using an anti-M3-muscarinic receptor monoclonal antibody and the immunoprecipitate probed in a Western blot using anti-phosphoserine384 antibody. The blot was then stripped and reprobed with a rabbit polyclonal anti-receptor antibody as a loading control. A typical experiment is shown together with the quantification from three independent experiments. (B) Wild-type mice were subjected to fear conditioning training, and the animals were killed either immediately after training (time point zero) or 5 min after training. The hippocampi were then dissected and membranes were prepared. Phosphorylation of the M3-muscarinic receptor using anti-phosphoserine384 antibody was then determined as in A. As a control for the stress response to the footshock, animals were subjected to immediate footshock with no fear conditioning training. Under these conditions there was no significant change in the phosphorylation status of serine 384 (Right). Typical experiments are shown together with the quantification from three independent experiments. The graphical data represent the means ± SE of three independent experiments. *, P < 0.05 (t test).
Similar articles
- M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase D1.
Kong KC, Butcher AJ, McWilliams P, Jones D, Wess J, Hamdan FF, Werry T, Rosethorne EM, Charlton SJ, Munson SE, Cragg HA, Smart AD, Tobin AB. Kong KC, et al. Proc Natl Acad Sci U S A. 2010 Dec 7;107(49):21181-6. doi: 10.1073/pnas.1011651107. Epub 2010 Nov 15. Proc Natl Acad Sci U S A. 2010. PMID: 21078968 Free PMC article. - Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor.
Nakajima K, Wess J. Nakajima K, et al. Mol Pharmacol. 2012 Oct;82(4):575-82. doi: 10.1124/mol.112.080358. Epub 2012 Jul 20. Mol Pharmacol. 2012. PMID: 22821234 Free PMC article. - M1 muscarinic receptor signaling in mouse hippocampus and cortex.
Porter AC, Bymaster FP, DeLapp NW, Yamada M, Wess J, Hamilton SE, Nathanson NM, Felder CC. Porter AC, et al. Brain Res. 2002 Jul 19;944(1-2):82-9. doi: 10.1016/s0006-8993(02)02721-x. Brain Res. 2002. PMID: 12106668 - Involvement of the cholinergic system in conditioning and perceptual memory.
Robinson L, Platt B, Riedel G. Robinson L, et al. Behav Brain Res. 2011 Aug 10;221(2):443-65. doi: 10.1016/j.bbr.2011.01.055. Epub 2011 Feb 17. Behav Brain Res. 2011. PMID: 21315109 Review. - Metabolic roles of the M3 muscarinic acetylcholine receptor studied with M3 receptor mutant mice: a review.
Gautam D, Jeon J, Li JH, Han SJ, Hamdan FF, Cui Y, Lu H, Deng C, Gavrilova O, Wess J. Gautam D, et al. J Recept Signal Transduct Res. 2008;28(1-2):93-108. doi: 10.1080/10799890801942002. J Recept Signal Transduct Res. 2008. PMID: 18437633 Review.
Cited by
- Gene expression analysis following olfactory learning in Apis mellifera.
Wang ZL, Wang H, Qin QH, Zeng ZJ. Wang ZL, et al. Mol Biol Rep. 2013 Feb;40(2):1631-9. doi: 10.1007/s11033-012-2212-9. Epub 2012 Oct 17. Mol Biol Rep. 2013. PMID: 23073783 - Modulation of Muscarinic Signalling in the Central Nervous System by Steroid Hormones and Neurosteroids.
Szczurowska E, Szánti-Pintér E, Chetverikov N, Randáková A, Kudová E, Jakubík J. Szczurowska E, et al. Int J Mol Sci. 2022 Dec 28;24(1):507. doi: 10.3390/ijms24010507. Int J Mol Sci. 2022. PMID: 36613951 Free PMC article. Review. - Neuromodulators and Long-Term Synaptic Plasticity in Learning and Memory: A Steered-Glutamatergic Perspective.
Bazzari AH, Parri HR. Bazzari AH, et al. Brain Sci. 2019 Oct 31;9(11):300. doi: 10.3390/brainsci9110300. Brain Sci. 2019. PMID: 31683595 Free PMC article. Review. - Muscarinic acetylcholine receptors act in synergy to facilitate learning and memory.
Leaderbrand K, Chen HJ, Corcoran KA, Guedea AL, Jovasevic V, Wess J, Radulovic J. Leaderbrand K, et al. Learn Mem. 2016 Oct 17;23(11):631-638. doi: 10.1101/lm.043133.116. Print 2016 Nov. Learn Mem. 2016. PMID: 27918283 Free PMC article. - Biased M1-muscarinic-receptor-mutant mice inform the design of next-generation drugs.
Bradley SJ, Molloy C, Valuskova P, Dwomoh L, Scarpa M, Rossi M, Finlayson L, Svensson KA, Chernet E, Barth VN, Gherbi K, Sykes DA, Wilson CA, Mistry R, Sexton PM, Christopoulos A, Mogg AJ, Rosethorne EM, Sakata S, John Challiss RA, Broad LM, Tobin AB. Bradley SJ, et al. Nat Chem Biol. 2020 Mar;16(3):240-249. doi: 10.1038/s41589-019-0453-9. Epub 2020 Feb 20. Nat Chem Biol. 2020. PMID: 32080630 Free PMC article.
References
- Anagnostaras SG, et al. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–58. - PubMed
- Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117:232–243. - PubMed
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. - PubMed
- McKinney M, Jacksonville MC. Brain cholinergic vulnerability: relevance to behavior and disease. Biochem Pharmacol. 2005;70:1115–1124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous