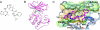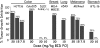Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth - PubMed (original) (raw)
. 2010 May 18;107(20):9446-51.
doi: 10.1073/pnas.0911863107. Epub 2010 May 3.
Chuangxing Guo, Joseph Piraino, John K Westwick, Cathy Zhang, Jane Lamerdin, Eleanor Dagostino, Daniel Knighton, Cho-Ming Loi, Michael Zager, Eugenia Kraynov, Ian Popoff, James G Christensen, Ricardo Martinez, Susan E Kephart, Joseph Marakovits, Shannon Karlicek, Simon Bergqvist, Tod Smeal
Affiliations
- PMID: 20439741
- PMCID: PMC2889050
- DOI: 10.1073/pnas.0911863107
Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth
Brion W Murray et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Despite abundant evidence that aberrant Rho-family GTPase activation contributes to most steps of cancer initiation and progression, there is a dearth of inhibitors of their effectors (e.g., p21-activated kinases). Through high-throughput screening and structure-based design, we identify PF-3758309, a potent (K(d) = 2.7 nM), ATP-competitive, pyrrolopyrazole inhibitor of PAK4. In cells, PF-3758309 inhibits phosphorylation of the PAK4 substrate GEF-H1 (IC(50) = 1.3 nM) and anchorage-independent growth of a panel of tumor cell lines (IC(50) = 4.7 +/- 3 nM). The molecular underpinnings of PF-3758309 biological effects were characterized using an integration of traditional and emerging technologies. Crystallographic characterization of the PF-3758309/PAK4 complex defined determinants of potency and kinase selectivity. Global high-content cellular analysis confirms that PF-3758309 modulates known PAK4-dependent signaling nodes and identifies unexpected links to additional pathways (e.g., p53). In tumor models, PF-3758309 inhibits PAK4-dependent pathways in proteomic studies and regulates functional activities related to cell proliferation and survival. PF-3758309 blocks the growth of multiple human tumor xenografts, with a plasma EC(50) value of 0.4 nM in the most sensitive model. This study defines PAK4-related pathways, provides additional support for PAK4 as a therapeutic target with a unique combination of functions (apoptotic, cytoskeletal, cell-cycle), and identifies a potent, orally available small-molecule PAK inhibitor with significant promise for the treatment of human cancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Structural characterization of PF-3758309 binding to the PAK4 catalytic domain. (A) Chemical structure of PF-3758309. (B) PF-3758309 in 2.1 Å PAK4 catalytic domain x-ray cocrystal structure (Protein Data Bank ID code 2x4z). (C) Binding of PF-3758309 in the active site of PAK4 (surface color-coded by hydrophobicity).
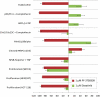
Fig. 2.
PF-3758309 has a unique profile of cellular activity. Cellular analysis was performed using a panel of over 113 cell-based assays, composed of both high-content and functional readouts, most performed at multiple time points. The group of assays shown was consistently regulated by diverse PAK4 inhibitors. Displayed in red is the core assay profile for PF-3758309 (1 μM); the Src-family kinase inhibitor Dasatinib (1 μM; green bars) is included for comparison. Each bar represents percent of inhibition stimulation relative to assay-specific controls from at least three independent experiments, ± SD (
SI Materials and Methods
).
Fig. 3.
Tumor growth inhibition of human xenograft tumor models: HCT116 (CRC), M24met (melanoma), Colo205 (CRC), A549 lung carcinoma, GTL-16 (GIST), DLD1 (CRC), and MDA-MB231 (BC). All TGI measurements were statistically significant except 20 mg/kg dosing of the c-Met-driven GTL-16 model (45).
Fig. 4.
PF-3758309 is antiproliferative and induces apoptosis in a HCT116 tumor model. PF-3758309 functional activity was measured by IHC in an HCT116 model with Ki67 expression and caspase 3 activation endpoints. (A) Ki67 and caspase 3 activation IHC analysis at 25 mg/kg PF-3758309. (B and C) Quantitation of the IHC images. Tumor-bearing mice were p.o. administered with vehicle (solid bars) and 15 mg/kg (open bars) and 25 mg/kg (striped bars) PF-3758309 twice daily and tumors were harvested at the indicated times.
Similar articles
- Inhibition of neuroblastoma proliferation by PF-3758309, a small-molecule inhibitor that targets p21-activated kinase 4.
Li Z, Li X, Xu L, Tao Y, Yang C, Chen X, Fang F, Wu Y, Ding X, Zhao H, Li M, Qian G, Xu Y, Ren J, Du W, Wang J, Lu J, Hu S, Pan J. Li Z, et al. Oncol Rep. 2017 Nov;38(5):2705-2716. doi: 10.3892/or.2017.5989. Epub 2017 Sep 22. Oncol Rep. 2017. PMID: 29048629 Free PMC article. - Targeting PAK4 Inhibits Ras-Mediated Signaling and Multiple Oncogenic Pathways in High-Risk Rhabdomyosarcoma.
Dasgupta A, Sierra L, Tsang SV, Kurenbekova L, Patel T, Rajapakse K, Shuck RL, Rainusso N, Landesman Y, Unger T, Coarfa C, Yustein JT. Dasgupta A, et al. Cancer Res. 2021 Jan 1;81(1):199-212. doi: 10.1158/0008-5472.CAN-20-0854. Epub 2020 Nov 9. Cancer Res. 2021. PMID: 33168646 Free PMC article. - Combined LIM kinase 1 and p21-Activated kinase 4 inhibitor treatment exhibits potent preclinical antitumor efficacy in breast cancer.
Zhao CC, Zhan MN, Liu WT, Jiao Y, Zhang YY, Lei Y, Zhang TT, Zhang CJ, Du YY, Gu KS, Wei W. Zhao CC, et al. Cancer Lett. 2020 Nov 28;493:120-127. doi: 10.1016/j.canlet.2020.08.006. Epub 2020 Aug 21. Cancer Lett. 2020. PMID: 32829006 - P21-activated kinase 4--not just one of the PAK.
Dart AE, Wells CM. Dart AE, et al. Eur J Cell Biol. 2013 Apr-May;92(4-5):129-38. doi: 10.1016/j.ejcb.2013.03.002. Epub 2013 Apr 4. Eur J Cell Biol. 2013. PMID: 23642861 Review. - Regulation of KRAS-PAK4 axis by microRNAs in cancer.
Choudhry ZS, Tripathi V, Sutton M, Bao B, Mohammad RM, Azmi AS. Choudhry ZS, et al. Curr Pharm Des. 2014;20(33):5275-8. doi: 10.2174/1381612820666140128203452. Curr Pharm Des. 2014. PMID: 24479809 Review.
Cited by
- Optimization of a Dibenzodiazepine Hit to a Potent and Selective Allosteric PAK1 Inhibitor.
Karpov AS, Amiri P, Bellamacina C, Bellance MH, Breitenstein W, Daniel D, Denay R, Fabbro D, Fernandez C, Galuba I, Guerro-Lagasse S, Gutmann S, Hinh L, Jahnke W, Klopp J, Lai A, Lindvall MK, Ma S, Möbitz H, Pecchi S, Rummel G, Shoemaker K, Trappe J, Voliva C, Cowan-Jacob SW, Marzinzik AL. Karpov AS, et al. ACS Med Chem Lett. 2015 May 22;6(7):776-81. doi: 10.1021/acsmedchemlett.5b00102. eCollection 2015 Jul 9. ACS Med Chem Lett. 2015. PMID: 26191365 Free PMC article. - RhoJ modulates melanoma invasion by altering actin cytoskeletal dynamics.
Ho H, Soto Hopkin A, Kapadia R, Vasudeva P, Schilling J, Ganesan AK. Ho H, et al. Pigment Cell Melanoma Res. 2013 Mar;26(2):218-25. doi: 10.1111/pcmr.12058. Epub 2013 Jan 7. Pigment Cell Melanoma Res. 2013. PMID: 23253891 Free PMC article. - Recent advances in the development of p21-activated kinase inhibitors.
Coleman N, Kissil J. Coleman N, et al. Cell Logist. 2012 Apr 1;2(2):132-135. doi: 10.4161/cl.21667. Cell Logist. 2012. PMID: 23162744 Free PMC article. - Targeting super-enhancer-associated oncogenes in oesophageal squamous cell carcinoma.
Jiang YY, Lin DC, Mayakonda A, Hazawa M, Ding LW, Chien WW, Xu L, Chen Y, Xiao JF, Senapedis W, Baloglu E, Kanojia D, Shang L, Xu X, Yang H, Tyner JW, Wang MR, Koeffler HP. Jiang YY, et al. Gut. 2017 Aug;66(8):1358-1368. doi: 10.1136/gutjnl-2016-311818. Epub 2016 May 10. Gut. 2017. PMID: 27196599 Free PMC article. - The Redox Role of G6PD in Cell Growth, Cell Death, and Cancer.
Yang HC, Wu YH, Yen WC, Liu HY, Hwang TL, Stern A, Chiu DT. Yang HC, et al. Cells. 2019 Sep 8;8(9):1055. doi: 10.3390/cells8091055. Cells. 2019. PMID: 31500396 Free PMC article. Review.
References
- Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. - PubMed
- Onesto C, Shutes A, Picard V, Schweighoffer F, Der CJ. Characterization of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. Methods Enzymol. 2008;439:111–129. - PubMed
- Zohn IM, Campbell SL, Khosravi-Far R, Rossman KL, Der CJ. Rho family proteins and Ras transformation: The RHOad less traveled gets congested. Oncogene. 1998;17(11 Reviews):1415–1438. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous