Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat - PubMed (original) (raw)
Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat
Qun Li et al. Neurosci Lett. 2010.
Abstract
Endogenous tri-potential neural stem cells (eNSCs) exist in the adult spinal cord and differentiate primarily into oligodendrocytes (OLs) and astrocytes. Previous in vivo and in vitro studies have shown that during development proliferation and differentiation of oligodendrocyte progenitor cells (OPCs) depend on activity in neighboring axons. However, this activity-dependent development of OPCs has not been examined in the adult CNS. In the present study, we stimulated unilateral corticospinal (CS) axons of the adult rat and investigated proliferation and differentiation of OPCs in dorsal corticospinal tract (dCST). eNSCs were labeled with the mitotic indicator 5-bromo-2'-deoxyuridine (BrdU). Phenotypes of proliferating cells were identified by double-immunolabeling of BrdU with a panel of antibodies to cell markers: NG2, Nkx2.2, APC, GFAP, and Glut-1. Electrical stimulation of CS axons increased BrdU labeled eNSCs and promoted the proliferation and differentiation of OPCs, but not astrocytes and endothelial cells. Our findings demonstrate the importance of neural activity in regulating OPC proliferation/differentiation in the mature CNS. Selective pathway electrical stimulation could be used to promote remyelination and recovery of function in CNS injury and disease.
Copyright 2010 Elsevier Ireland Ltd. All rights reserved.
Figures
Fig 1
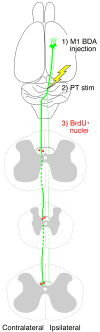
Experimental design. BDA was injected unilaterally into cortex to label the CST (green). Two weeks later, the PT electrode was implanted and stimulated daily (10 days; yellow). During the stimulation period, BrdU was administrated every other day. BrdU+ cells in dCST were counted (red).
Fig 2
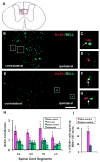
BDA+ CST axons (green) and BrdU+ nuclei (red) in electrically stimulated animals. A: Coronal spinal cord section. More than 95% BDA labeled CST axons project in the contralateral dCST. The red box indicates the micrographs in B and E. B: T6 section. C and D: higher magnification of boxed regions in B. BrdU+ nuclei distributed predominantly in contralateral dCST and most of them apposed BDA+ axons (C; arrow). D, BrdU+ cell not contacting a BDA+ axon. E: L3 section. Parts F and G, higher magnification of boxed regions in E. Sparse BDA+ axons in the ipsilateral dCST always closely apposed BrdU+ cells (arrows). Bar, 50 μm. H: BrdU+ nuclei in the dCST of the four groups. Number of labeled cells in experimental group (contralateral dCST of stimulated animals) was significantly greater than the three control groups (two-way ANOVA; *: p< 0.0001). I: There was a significantly higher percentage of BrdU+ cells contacting BDA+ axons in the contralateral dCST than in sham animals (t-test; *: p<0.01).
Fig 3
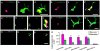
Double immunolabeled BrdU+ cells and different markers in contralateral dCST in stimulated animals. A, B, C: a NG2 stained BrdU+ OPC. D, E, F: a dividing BrdU+/APC+ mature oligodendrocyte. Arrows indicate processes of NG2+ and APC+ cells apposing BDA labeled axons. G, H, I, J: A pair of BrdU and Nkx-2.2 double-labeled nuclei contacting a stimulated axon. J. Higher magnification of box in I. K, L, M: a BrdU+/GFAP+ proliferating astrocyte. N, O, P: BrdU+/Glut-1+ cell. All bars in figures, 10 μm. Q: Bar graphs showing that numbers of BrdU+/NG2+, BrdU+/APC+, and BrdU+/Nkx-2.2+ cells in the experimental group are significantly greater then control and that there is no statistical difference between experimental and control groups for BrdU+/GFAP+ and BrdU+/Glut-1+ cells (t-test; *: p < 0.05; **: p<0.01).
Fig 4
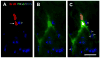
A BrdU+ cell in the stimulated group asymmetrically divided into two daughter cells. One cell (arrow in A) contacted labeled/stimulated dCST axons (open arrow in A) and differentiated into an NG2+ OPC (B). The sister cell, which did not contact dCST axons, did not develop this phenotype. Small open arrows in C indicate multiple appositions between stimulated axons and cell body or dendrites of the OPC. No asymmetrical divisions were found in control groups. Bar, 10 μm
Similar articles
- Induced Neural Activity Promotes an Oligodendroglia Regenerative Response in the Injured Spinal Cord and Improves Motor Function after Spinal Cord Injury.
Li Q, Houdayer T, Liu S, Belegu V. Li Q, et al. J Neurotrauma. 2017 Dec 15;34(24):3351-3361. doi: 10.1089/neu.2016.4913. Epub 2017 Aug 10. J Neurotrauma. 2017. PMID: 28474539 - Protective Effect of Electroacupuncture on Neural Myelin Sheaths is Mediated via Promotion of Oligodendrocyte Proliferation and Inhibition of Oligodendrocyte Death After Compressed Spinal Cord Injury.
Huang S, Tang C, Sun S, Cao W, Qi W, Xu J, Huang J, Lu W, Liu Q, Gong B, Zhang Y, Jiang J. Huang S, et al. Mol Neurobiol. 2015 Dec;52(3):1870-1881. doi: 10.1007/s12035-014-9022-0. Epub 2014 Dec 4. Mol Neurobiol. 2015. PMID: 25465241 - Astrocytes regulate the expression of Sp1R3 on oligodendrocyte progenitor cells through Cx47 and promote their proliferation.
Xu D, Liu Z, Wang S, Peng Y, Sun X. Xu D, et al. Biochem Biophys Res Commun. 2017 Aug 26;490(3):670-675. doi: 10.1016/j.bbrc.2017.06.099. Epub 2017 Jun 17. Biochem Biophys Res Commun. 2017. PMID: 28634078 - Function of Lymphocytes in Oligodendrocyte Development.
Tanabe S, Yamashita T. Tanabe S, et al. Neuroscientist. 2020 Feb;26(1):74-86. doi: 10.1177/1073858419834221. Epub 2019 Mar 8. Neuroscientist. 2020. PMID: 30845892 Review. - Engineering biomaterial microenvironments to promote myelination in the central nervous system.
Unal DB, Caliari SR, Lampe KJ. Unal DB, et al. Brain Res Bull. 2019 Oct;152:159-174. doi: 10.1016/j.brainresbull.2019.07.013. Epub 2019 Jul 12. Brain Res Bull. 2019. PMID: 31306690 Review.
Cited by
- Myelin plasticity: sculpting circuits in learning and memory.
Xin W, Chan JR. Xin W, et al. Nat Rev Neurosci. 2020 Dec;21(12):682-694. doi: 10.1038/s41583-020-00379-8. Epub 2020 Oct 12. Nat Rev Neurosci. 2020. PMID: 33046886 Free PMC article. Review. - Chronic muscle recordings reveal recovery of forelimb function in spinal injured female rats after cortical epidural stimulation combined with rehabilitation and chondroitinase ABC.
Sinopoulou E, Spejo AB, Roopnarine N, Burnside ER, Bartus K, De Winter F, McMahon SB, Bradbury EJ. Sinopoulou E, et al. J Neurosci Res. 2022 Nov;100(11):2055-2076. doi: 10.1002/jnr.25111. Epub 2022 Aug 2. J Neurosci Res. 2022. PMID: 35916483 Free PMC article. - On Myelinated Axon Plasticity and Neuronal Circuit Formation and Function.
Almeida RG, Lyons DA. Almeida RG, et al. J Neurosci. 2017 Oct 18;37(42):10023-10034. doi: 10.1523/JNEUROSCI.3185-16.2017. J Neurosci. 2017. PMID: 29046438 Free PMC article. Review. - Low-intensity transcranial magnetic stimulation promotes the survival and maturation of newborn oligodendrocytes in the adult mouse brain.
Cullen CL, Senesi M, Tang AD, Clutterbuck MT, Auderset L, O'Rourke ME, Rodger J, Young KM. Cullen CL, et al. Glia. 2019 Aug;67(8):1462-1477. doi: 10.1002/glia.23620. Epub 2019 Apr 16. Glia. 2019. PMID: 30989733 Free PMC article. - NG2 cells (polydendrocytes): listeners to the neural network with diverse properties.
Hill RA, Nishiyama A. Hill RA, et al. Glia. 2014 Aug;62(8):1195-210. doi: 10.1002/glia.22664. Epub 2014 Apr 21. Glia. 2014. PMID: 24753030 Free PMC article. Review.
References
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells dependents on electrical activity in axons. Nature. 1993;361:258–260. - PubMed
- Belci M, Catley M, Husain M, Frankel HL, Davey NJ. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord. 2004;42:417–419. - PubMed
- Bruce W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci. 2003;206:181–185. 2003. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous