Pom33, a novel transmembrane nucleoporin required for proper nuclear pore complex distribution - PubMed (original) (raw)
Pom33, a novel transmembrane nucleoporin required for proper nuclear pore complex distribution
Anne Chadrin et al. J Cell Biol. 2010.
Abstract
The biogenesis of nuclear pore complexes (NPCs) represents a paradigm for the assembly of high-complexity macromolecular structures. So far, only three integral pore membrane proteins are known to function redundantly in NPC anchoring within the nuclear envelope. Here, we describe the identification and functional characterization of Pom33, a novel transmembrane protein dynamically associated with budding yeast NPCs. Pom33 becomes critical for yeast viability in the absence of a functional Nup84 complex or Ndc1 interaction network, which are two core NPC subcomplexes, and associates with the reticulon Rtn1. Moreover, POM33 loss of function impairs NPC distribution, a readout for a subset of genes required for pore biogenesis, including members of the Nup84 complex and RTN1. Consistently, we show that Pom33 is required for normal NPC density in the daughter nucleus and for proper NPC biogenesis and/or stability in the absence of Nup170. We hypothesize that, by modifying or stabilizing the nuclear envelope-NPC interface, Pom33 may contribute to proper distribution and/or efficient assembly of nuclear pores.
Figures
Figure 1.
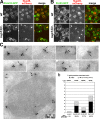
Pom33, and to a lower extent its paralogue Per33, are associated with NPCs. (A and B) Fluorescence microscopy analysis of Pom33-GFP (A) and Per33-GFP (B) in wt and nup133Δ cells expressing Nup49-mCherry. Spinning disk confocal images of single-channel fluorescence for GFP and mCherry are shown (left) as well as the merge (right). Bar, 5 µm. (C) Immunolocalization of GFP-tagged Pom33 at nuclear pores. Cryosections of Pom33-GFP cells were successively labeled with anti-GFP (detected using ProtA 10-nm gold particles) and with mAb414 (detected using ProtA 15-nm gold particles). (a) Typical patterns of Pom33-GFP localization at NPCs (detected based on NE structure and mAb414 labeling) as well as a section encompassing a nucleus are presented. Arrows point to 10-nm gold particles recognizing the GFP moiety of Pom33-GFP at nuclear pores. Arrowheads point to 10-nm gold particles localized outside of the NE. The cytoplasmic faces of the NE are oriented toward the top of each micrograph. (b) Statistical analysis of the distribution of anti-GFP–associated gold particles in control (expressing no GFP fusion protein), Pom33-GFP, Per33-GFP, and Ndc1-GFP cells. For each strain, the total number of anti-GFP–associated gold particles (n1) and of cryosectioned cells (n2) analyzed, as well as the percentage of GFP-associated gold particles localized at NPCs relative to the entire NE (NPC + NE), are indicated.
Figure 2.
Pom33 is a dynamic component of nuclear pores. (A) FLIP analysis in Ndc1-GFP–, Pom33-GFP–, Per33-GFP–, and Sec61-GFP–expressing cells. Shown are confocal microscopy images of representative cells before photobleaching and after ∼15–20 bleaching pulses (100 s). The cell shape is outlined in white and the bleached area within the cortical ER is outlined in yellow. Bar, 2 µm. (B) For each strain, the fluorescence decay in the nucleus (closed symbols) and in the ER (open symbols) was quantified as described in Materials and methods. Each curve represents the mean of a least 16 independent cells. SD is also indicated. (C) For each cell, the ratio of fluorescence in the nucleus over the ER, normalized to 1 at t = 0, was quantified at each time point. The median value and SD (error bars) are plotted over time.
Figure 3.
S. cerevisiae Pom33 and Per33 and human TMEM33 are evolutionarily conserved NE/ER-associated proteins. (A) Phylogenic tree of S. cerevisiae Pom33 and Per33 homologues. The tree, based on ClustalW alignment of sequences identified by BLAST searches using ScPom33, ScPer33, and HsTMEM33 (highlighted in bold), was constructed with the Jalview software (
; Waterhouse et al., 2009) based on the percentage of identity between the proteins. (B) ClustalW alignment of ScPom33, ScPer33, and HsTMEM33. Identical residues are colored in black, and similar residues are colored in gray. Asterisks in the consensus line denote residues that are identical in all three proteins sequences, and periods indicate residues that are either identical or similar in at least two out of the three sequences. Approximate positions of the hydrophobic stretches are shown on top of the alignment (black line, see also
Fig. S1 C
).
Figure 4.
POM33 displays genetic interactions with core components of NPC. (A) Growth properties of wt_, pom33Δ, per33Δ, nup133Δ_, and of corresponding double and triple mutants. Equivalent amounts of cells were spotted as fivefold dilutions on YEPD plates, and were incubated for 5 d at 18°C or for 3 d at 25, 30, and 37°C. (B) Summary of the genetic interactions between pom33Δ, per33Δ, or pom33Δ per33Δ, and Nups or NE/ER mutants. Synthetic interactions were scored as: SS, strong synergistic interaction; S, synergistic interaction; V, viable; n.d., not determined. The temperature above which the synthetic phenotype was observed is indicated. Footnote a, see Fig. 4 A; footnote b, see
Fig. S2
; *, note that PER33 deletion partially rescues the pom33Δ nup133Δ synthetic phenotype at 18°C. (C) Growth assay of ndc1-39 mutant cells transformed with an empty pRS315 plasmid (ø), with centromeric (cen), or with multicopy (2µ) plasmids encompassing the POM33 or PER33 genes. Transformants were spotted as fivefold dilutions on selective medium, and plates were incubated for 3 d at the indicated temperatures.
Figure 5.
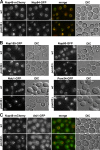
Pom33 interacts with the Rtn protein Rtn1. (A) Affinity purification by IgG chromatography of Pom33-ProtA in Rtn1-GFP–, rtn1-K48I-GFP–, or Yop1-GFP–expressing strains. Total soluble extracts (inputs) and affinity-purified fractions (eluates, 2.5-fold equivalent for the anti–[α]-ProtA and 2,500-fold equivalent for the other antibodies) from strains expressing (+) or not expressing (−) Pom33-ProtA were analyzed by Western blotting using the indicated antibodies. Dpm1 and the nucleolar protein Nop1 were used as controls. (B) Spinning disk confocal images of rtn1Δ nup133Δ cells expressing Rtn1-GFP or rtn1K48I-GFP and Nup49-mCherry. Note that unlike wt Rtn1, the rtn1-K48I mutant is not enriched at the clustered pores labeled with Nup49-mCherry. Bar, 5 µm. (C) Affinity purification by IgG chromatography of Pom33-ProtA or Pom34-ProtA in Rtn1-GFP–expressing cells. Total soluble extracts (inputs) and affinity-purified fractions (eluates, fivefold equivalent for the anti-ProtA and 2,500-fold equivalent for the other antibodies) were analyzed by Western blotting using the indicated antibodies. Black lines indicate that intervening lanes have been spliced out. Size markers on the sides of the gel blots indicate kD.
Figure 6.
The pom33Δ mutant displays an NPC clustering phenotype. (A) The localization of Nup84-GFP was analyzed in wt and pom33Δ cells carrying the Nup49-mCherry plasmid. Wide-field images of single-channel fluorescence for mCherry and GFP; merge and DIC images are also shown. (B) The localization of the indicated GFP-tagged Nups was analyzed by direct fluorescence microscopy in wt and pom33Δ cells. The bright Ndc1-GFP dots in wt and pom33Δ cells correspond to the spindle pole body. (C) Fluorescence microscopy analysis of the localization of the inner nuclear membrane protein Asi1-GFP in wt and pom33Δ cells expressing Nup49-mCherry. Bars, 5 µm.
Figure 7.
Thin-section EM analysis of the NE and NPCs in wt and pom33Δ cells. wt cells (A–C) and pom33Δ cells (D–J) were grown to early log phase at 30°C and processed for thin-section EM (as described in Materials and methods). Arrowheads point to typical NPCs and asterisks denote distorted NPC-like structures. Bar, 500 nm.
Figure 8.
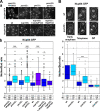
Pom33 is required for normal pore density in the daughter nucleus in telophase. (A, a) Nup84-GFP fluorescence was analyzed by confocal microscopy in the indicated yeast strains. 3D reconstructions of total fluorescence from 13 planes covering the entire nuclei are shown. The cell shape of dividing cells is outlined in white. Bar, 5 µm. (b) The ratios of the total fluorescence intensity of Nup84-GFP in the mother versus the bud nuclei, quantified in telophase cells, are represented as box plots using KaleidaGraph (see Materials and methods). Mean values (m) and SD of the M/B ratios are indicated below the corresponding strains. *, 10−10 < P < 5 × 10−3 compared with wt using the Student’s t test; ***, P < 10−10. The total number of cells quantified is indicated (n; arising from at least two independent experiments). (B) M/B ratio distribution of Nup84-GFP fluorescence in wt and pom33Δ cells at early anaphase, telophase, and G1 stages of mitosis was plotted as in A. Confocal images of Nup84-GFP at the corresponding stages, revealed by the cell shape outlined in white, are shown on top. Bar, 2 µm.
Figure 9.
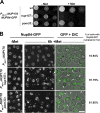
POM33 deletion impairs Nup84-GFP incorporation into NPCs upon Nup170 depletion. (A) Growth properties of wt, nup157Δ, and pom33Δ cells expressing NUP170 under the control of the PMET3 repressible promoter in a NUP84-GFP background. Serial dilutions were spotted on selective media lacking methionine (−Met) or on YEPD (+Met). (B) The localization of Nup84-GFP was analyzed in the indicated strains by spinning disk confocal microscopy before (−Met) or after a 6-h repression of NUP170 expression (+Met). Cytoplasmic Nup84-GFP foci are observed upon repression of NUP170 in nup157Δ and to a lower extent in pom33Δ cells (arrows, foci in mother cells; arrowheads, foci in buds). The percentage of cells with Nup84-GFP cytoplasmic foci is indicated. Bar, 5 µm.
Similar articles
- Nuclear pore targeting of the yeast Pom33 nucleoporin depends on karyopherin and lipid binding.
Floch AG, Tareste D, Fuchs PF, Chadrin A, Naciri I, Léger T, Schlenstedt G, Palancade B, Doye V. Floch AG, et al. J Cell Sci. 2015 Jan 15;128(2):305-16. doi: 10.1242/jcs.158915. Epub 2014 Nov 20. J Cell Sci. 2015. PMID: 25413348 - ER membrane-bending proteins are necessary for de novo nuclear pore formation.
Dawson TR, Lazarus MD, Hetzer MW, Wente SR. Dawson TR, et al. J Cell Biol. 2009 Mar 9;184(5):659-75. doi: 10.1083/jcb.200806174. J Cell Biol. 2009. PMID: 19273614 Free PMC article. - Integrity and function of the Saccharomyces cerevisiae spindle pole body depends on connections between the membrane proteins Ndc1, Rtn1, and Yop1.
Casey AK, Dawson TR, Chen J, Friederichs JM, Jaspersen SL, Wente SR. Casey AK, et al. Genetics. 2012 Oct;192(2):441-55. doi: 10.1534/genetics.112.141465. Epub 2012 Jul 13. Genetics. 2012. PMID: 22798490 Free PMC article. - Nuclear pore complex biogenesis.
Fernandez-Martinez J, Rout MP. Fernandez-Martinez J, et al. Curr Opin Cell Biol. 2009 Aug;21(4):603-12. doi: 10.1016/j.ceb.2009.05.001. Epub 2009 Jun 11. Curr Opin Cell Biol. 2009. PMID: 19524430 Free PMC article. Review. - Toward the atomic structure of the nuclear pore complex: when top down meets bottom up.
Hoelz A, Glavy JS, Beck M. Hoelz A, et al. Nat Struct Mol Biol. 2016 Jul;23(7):624-30. doi: 10.1038/nsmb.3244. Epub 2016 Jun 6. Nat Struct Mol Biol. 2016. PMID: 27273515 Free PMC article. Review.
Cited by
- Sm protein down-regulation leads to defects in nuclear pore complex disassembly and distribution in C. elegans embryos.
Joseph-Strauss D, Gorjánácz M, Santarella-Mellwig R, Voronina E, Audhya A, Cohen-Fix O. Joseph-Strauss D, et al. Dev Biol. 2012 May 15;365(2):445-57. doi: 10.1016/j.ydbio.2012.02.036. Epub 2012 Mar 8. Dev Biol. 2012. PMID: 22426005 Free PMC article. - Quantifying nucleoporin stoichiometry inside single nuclear pore complexes in vivo.
Mi L, Goryaynov A, Lindquist A, Rexach M, Yang W. Mi L, et al. Sci Rep. 2015 Mar 23;5:9372. doi: 10.1038/srep09372. Sci Rep. 2015. PMID: 25797490 Free PMC article. - Sumoylation of the THO complex regulates the biogenesis of a subset of mRNPs.
Bretes H, Rouviere JO, Leger T, Oeffinger M, Devaux F, Doye V, Palancade B. Bretes H, et al. Nucleic Acids Res. 2014 Apr;42(8):5043-58. doi: 10.1093/nar/gku124. Epub 2014 Feb 5. Nucleic Acids Res. 2014. PMID: 24500206 Free PMC article. - Chm7 and Heh1 collaborate to link nuclear pore complex quality control with nuclear envelope sealing.
Webster BM, Thaller DJ, Jäger J, Ochmann SE, Borah S, Lusk CP. Webster BM, et al. EMBO J. 2016 Nov 15;35(22):2447-2467. doi: 10.15252/embj.201694574. Epub 2016 Oct 12. EMBO J. 2016. PMID: 27733427 Free PMC article. - ESCRTs breach the nuclear border.
Webster BM, Lusk CP. Webster BM, et al. Nucleus. 2015;6(3):197-202. doi: 10.1080/19491034.2015.1035844. Epub 2015 May 5. Nucleus. 2015. PMID: 25942571 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases