NMDA-induced neuronal survival is mediated through nuclear factor I-A in mice - PubMed (original) (raw)
NMDA-induced neuronal survival is mediated through nuclear factor I-A in mice
Sika Zheng et al. J Clin Invest. 2010 Jul.
Abstract
Identification of the signaling pathways that mediate neuronal survival signaling could lead to new therapeutic targets for neurologic disorders and stroke. Sublethal doses of NMDA can induce robust endogenous protective mechanisms in neurons. Through differential analysis of primary library expression and microarray analyses, here we have shown that nuclear factor I, subtype A (NFI-A), a member of the NFI/CAAT-box transcription factor family, is induced in mouse neurons by NMDA receptor activation in a NOS- and ERK-dependent manner. Knockdown of NFI-A induction using siRNA substantially reduced the neuroprotective effects of sublethal doses of NMDA. Further analysis indicated that NFI-A transcriptional activity was required for the neuroprotective effects of NMDA receptor activation. Additional evidence of the neuroprotective effects of NFI-A was provided by the observations that Nfia(-/-) neurons were highly sensitive to NMDA-induced excitotoxicity and were more susceptible to developmental cell death than wild-type neurons and that Nfia(+/-) mice were more sensitive to NMDA-induced intrastriatal lesions than were wild-type animals. These results identify NFI-A as what we believe to be a novel neuroprotective transcription factor with implications in neuroprotection and neuronal plasticity following NMDA receptor activation.
Figures
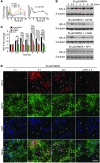
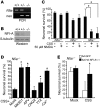
Figure 1. NFI-A is induced by neuroprotective models in vitro.
(A) Induction of Nfia mRNA splice variants (left) and total Nfia mRNA (right) upon 50 μM NMDA (5 minutes) treatment in primary cortical cultures. Message levels were measured by quantitative real-time PCR using isoform-specific primer sets. Total Nfia mRNA was measured in at least 3 independent experiments, with mRNA levels normalized relative to Gapdh internal control. (B) Immunoblot analysis of the induction of NFI-A following a 5-minute 50 μM NMDA treatment of primary cortical cultures with or without the MEK inhibitor U0126 (50 μM), the NOS inhibitor nitro-
l
-arginine (L-NNA, 100 μM), or the NMDA receptor antagonist APV (250 μM) applied 30 minutes before 50 μM NMDA treatment. (C) Quantification of NFI-A levels was normalized to β-tubulin expression. Experiments were replicated at least 3 times; *P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA followed by Tukey-Kramer post-hoc test. (D) Immunocytochemical staining of cortical cultures at 0, 3, and 24 hours after 5-minute 50 μM NMDA treatment shows induction of NFI-A only in neurons (MAP2+), which is blocked by APV (250 μM). Note that the increased intensity of NFI-A staining in MAP2+ cells and staining in non-neuronal cells (arrows) does not change following NMDA treatment. These data are representative of 3 separate experiments. Scale bar: 50 μm.
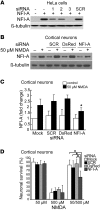
Figure 2. Blocking NFI-A induction by 50 μM NMDA neuroprotective treatment significantly inhibits its protective effects.
(A) Knockdown of ectopic NFI-A expression by NFI-A siRNAs (1, 2, and 3) in HeLa cells. SCR, scrambled control siRNA. siRNA and NFI-A expression plasmid were cotransfected into HeLa cells. Twenty-four hours after transfection, NFI-A expression levels were examined from total cell lysates. These experiments were replicated 3 times. (B) Immunoblot analysis of blockade of NFI-A induction by 50 μM NMDA treatment using NFI-A siRNA 3, but not by SCR siRNA or DsRed siRNA molecules. Cortical cultures were transfected with siRNA 1 day and 3 days prior to 50 μM NMDA treatment and were harvested 24 hours after NMDA treatment. Experiments were replicated 3 times. (C) Quantification of NFI-A levels. *P < 0.01, 1-way ANOVA followed by Tukey-Kramer post-hoc test. (D) Neuronal viability after 500 μM NMDA excitotoxicity in cortical cultures. siRNAs were transfected twice at 3 days and 1 day prior to 50 μM NMDA treatment. The cultures were challenged with 500 μM NMDA toxicity for 5 minutes at 24 hours after the 5-minute 50 μM NMDA treatment. Experiments were replicated at least 3 times, with at least 6,000 neurons counted per experiment. NFI-A siRNA treatment (right gray bar) significantly blocks the protective effects of 50 μM NMDA against 500 μM NMDA treatment. *P < 0.01, 1-way ANOVA followed by Tukey-Kramer post-hoc test.
Figure 3. NFI-A protects against a variety of toxic insults.
(A) Representative photomicrographs of cortical cultures infected with GFP adenovirus, NFI-A1.1 adenovirus, or NFI-A1.1B adenovirus and pretreated for 5 minutes with 50 μM NMDA or mock treated (CSS/CSS), then challenged with 500 μM NMDA excitotoxicity. (B) Quantification of neuronal viability. Experiments were replicated at least 4 times. *P < 0.01, 1-way ANOVA followed by Tukey-Kramer post-hoc test. Scale bar: 50 μm. (C) Neurons transformed with Ad.HA-NFI-A1.1 or Ad.GFP were treated with 100 μM kainate or 100 μM AMPA or (D) 300 μM H2O2. Experiments were replicated at least 3 times. *P < 0.01, Student’s t test, treated versus GFP-overexpressing neurons. (E) SCG neurons preinfected with GFP or NFI-A1.1 adenovirus were deprived of NGF. Experiments were replicated at least 4 times. *P < 0.01, 1-way ANOVA followed by Tukey-Kramer post-hoc test, compared with GFP control or mock control SCG neurons deprived of NGF.
Figure 4. NFI-A protects neurons through its transcriptional activity.
(A) Schematic representation of NFI luciferase reporter (NFI LUC), which was generated from the basic luciferase reporter (Tal LUC) by inserting triplicate NFI response elements (TTGGCACGGAGCCAA) upstream of the basal transcriptional Tal promoter. NFI-A DBM is a DNA-binding mutant with 3 cysteine residues (Cys2, Cys4, Cys5) in the DNA-binding domain mutated to serine residues. NFI-A DBD is a deletion mutant in which the whole DNA-binding domain was truncated. (B) Luciferase activity in neurons transfected with combinations of NFI-A, NFI-A mutants, or luciferase reporters. Experiments were replicated at least 3 times. *P < 0.01, 1-way ANOVA followed by Tukey-Kramer post-hoc test. (C) Luciferase activity following 50 μM NMDA treatment in primary cortical cultures transfected with NFI LUC or Tal LUC. Experiments were replicated at least 3 times. (D) Neuronal viability after 500 μM NMDA excitotoxicity treatment in primary cortical cultures transfected with NFI-A, NFI-A mutants, or control vector (pCHA) and cotransfected with a GFP plasmid. Experiments were replicated at least 4 times. *P < 0.01, 1-way ANOVA followed by Tukey-Kramer post-hoc test, compared with control vector (pCHA), NFI-A DBM, or NFI-A DBD. (E) Immunoblot analysis of NFI-A, NFI-A DBM, and NFI-A DBD showing similar levels of expression in SHSY5Y cells 24 hours after plasmid transfection. Experiments were replicated 3 times. (F) Quantification of immunoblot analysis in E by laser densitometry.
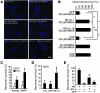
Figure 5. Delivery of NFI-A adenovirus into striatum followed by NMDA microinjection.
(A, B, E, F, I, and J) Ad.GFP or (C, D, G, H, K, and L) Ad.HA-NFI-A1.1 at 108 PFU was injected into mouse striatum. Three days after virus injection, the mice were subjected to a second microinjection of 0.3 ml PBS (A, C, E, G, I, and K) or 0.3 μl 50 mM NMDA (B, D, F, H, J, and L) into the same site as the original virus injection site. Two days afterward, 40-μM brain sections through the striatum were prepared and labeled with antibodies to NeuN, HA, or GFP. Scale bar: 50 μm. (M) Quantification of surviving neurons. In the striatum, the boundaries of the lesion area are clearly identified by observing the boundaries of NeuN staining. Every section through the lesion was analyzed, and GFP/NeuN double-positive or HA/NeuN double-positive cells were counted and quantified. For each group, n = 4. *P < 0.01, 1-way ANOVA followed by Tukey-Kramer post-hoc test, compared with GFP/NMDA-treated animals. **Not significantly different compared with GFP/PBS-treated animals.
Figure 6. NFI-A–deficient neurons are sensitive to NMDA receptor activation.
(A) Genotyping of embryos whose brains were used for cortical cultures. (B) NFI-A expression in day 10 in vitro neuronal cultures with different genotypes. (C) Cortical cultures dissociated from Nfia+/+, Nfia+/–, and Nfia–/– embryos were treated with either CSS or 50 μM NMDA. Experiments were replicated at least 3 times. *P < 0.01, 1-way ANOVA followed by Tukey-Kramer post-hoc test. (D) Nfia–/– neurons were treated with CSS with or without MK801 (10 μM), APV (250 μM), 6-cyano-7-nitroquinoxaline-2,3,-dione (CNQX) (400 μM), TTX (2 μM), or CSS with calcium replaced by cobalt (–Ca2+). *P < 0.01 compared with CSS mock treatment, 1-way ANOVA followed by Tukey-Kramer post-hoc test. Experiments were replicated 3 times. (E) The increased sensitivity of Nfia–/– neurons is rescued by Ad.HA-NFI-A1.1–mediated NFI-A overexpression in primary cortical cultures. Experiments were replicated 3 times. *P < 0.01, Student’s t test, compared with Nfia–/– neurons transduced with GFP adenovirus.
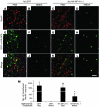
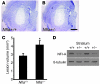
Figure 7. NFI-A–deficient neurons are sensitive to NMDA treatment in vivo.
(A and B) Representative photomicrograph of intrastriatal lesion of (A) Nfia+/+ mice and (B) Nfia+/– mice 2 days after microinjection of 0.3 ml NMDA (50 mM). Scale bar: 50 mm. (C) Quantification of lesion volumes. n = 4; *P < 0.01, Student’s t test. (D) Immunoblot of NFI-A expression in the striatum of Nfia+/+ and Nfia+/– mice.
Similar articles
- N-methyl-D-aspartate and TrkB receptor activation in cerebellar granule cells: an in vitro model of preconditioning to stimulate intrinsic survival pathways in neurons.
Jiang X, Zhu D, Okagaki P, Lipsky R, Wu X, Banaudha K, Mearow K, Strauss KI, Marini AM. Jiang X, et al. Ann N Y Acad Sci. 2003 May;993:134-45; discussion 159-60. doi: 10.1111/j.1749-6632.2003.tb07522.x. Ann N Y Acad Sci. 2003. PMID: 12853306 Free PMC article. Review. - N-methyl-D-aspartate and TrkB receptors protect neurons against glutamate excitotoxicity through an extracellular signal-regulated kinase pathway.
Zhu D, Wu X, Strauss KI, Lipsky RH, Qureshi Z, Terhakopian A, Novelli A, Banaudha K, Marini AM. Zhu D, et al. J Neurosci Res. 2005 Apr 1;80(1):104-13. doi: 10.1002/jnr.20422. J Neurosci Res. 2005. PMID: 15744743 Free PMC article. - A microscopy-based small molecule screen in primary neurons reveals neuroprotective properties of the FDA-approved anti-viral drug Elvitegravir.
Merz SF, Bengtson CP, Tepohl C, Hagenston AM, Bading H, Bas-Orth C. Merz SF, et al. Mol Brain. 2020 Sep 14;13(1):124. doi: 10.1186/s13041-020-00641-1. Mol Brain. 2020. PMID: 32928261 Free PMC article. - Neuroprotective activity of the mGluR5 antagonists MPEP and MTEP against acute excitotoxicity differs and does not reflect actions at mGluR5 receptors.
Lea PM 4th, Movsesyan VA, Faden AI. Lea PM 4th, et al. Br J Pharmacol. 2005 Jun;145(4):527-34. doi: 10.1038/sj.bjp.0706219. Br J Pharmacol. 2005. PMID: 15821750 Free PMC article. - [Protective effects of N-methyl-D-aspartate against neuronal damages].
Okuda H, Ogita K. Okuda H, et al. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002 Oct;22(5):153-8. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002. PMID: 12451685 Review. Japanese.
Cited by
- PSD-95 is post-transcriptionally repressed during early neural development by PTBP1 and PTBP2.
Zheng S, Gray EE, Chawla G, Porse BT, O'Dell TJ, Black DL. Zheng S, et al. Nat Neurosci. 2012 Jan 15;15(3):381-8, S1. doi: 10.1038/nn.3026. Nat Neurosci. 2012. PMID: 22246437 Free PMC article. - p38 MAP kinase-mediated NMDA receptor-dependent suppression of hippocampal hypersynchronicity in a mouse model of Alzheimer's disease.
Ittner AA, Gladbach A, Bertz J, Suh LS, Ittner LM. Ittner AA, et al. Acta Neuropathol Commun. 2014 Oct 21;2:149. doi: 10.1186/s40478-014-0149-z. Acta Neuropathol Commun. 2014. PMID: 25331068 Free PMC article. - All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class.
Castillo-Gómez E, Oliveira B, Tapken D, Bertrand S, Klein-Schmidt C, Pan H, Zafeiriou P, Steiner J, Jurek B, Trippe R, Prüss H, Zimmermann WH, Bertrand D, Ehrenreich H, Hollmann M. Castillo-Gómez E, et al. Mol Psychiatry. 2017 Dec;22(12):1776-1784. doi: 10.1038/mp.2016.125. Epub 2016 Aug 9. Mol Psychiatry. 2017. PMID: 27502473 - Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: implications for preventive treatments of ischemic stroke and late-onset Alzheimer's disease.
Yu SP, Jiang MQ, Shim SS, Pourkhodadad S, Wei L. Yu SP, et al. Mol Neurodegener. 2023 Jul 3;18(1):43. doi: 10.1186/s13024-023-00636-1. Mol Neurodegener. 2023. PMID: 37400870 Free PMC article. Review. - Integrated model of de novo and inherited genetic variants yields greater power to identify risk genes.
He X, Sanders SJ, Liu L, De Rubeis S, Lim ET, Sutcliffe JS, Schellenberg GD, Gibbs RA, Daly MJ, Buxbaum JD, State MW, Devlin B, Roeder K. He X, et al. PLoS Genet. 2013;9(8):e1003671. doi: 10.1371/journal.pgen.1003671. Epub 2013 Aug 15. PLoS Genet. 2013. PMID: 23966865 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous