Genetics and complement in atypical HUS - PubMed (original) (raw)
Review
Genetics and complement in atypical HUS
David Kavanagh et al. Pediatr Nephrol. 2010 Dec.
Abstract
Central to the pathogenesis of atypical hemolytic uremic syndrome (aHUS) is over-activation of the alternative pathway of complement. Following the initial discovery of mutations in the complement regulatory protein, factor H, mutations have been described in factor I, membrane cofactor protein and thrombomodulin, which also result in decreased complement regulation. Autoantibodies to factor H have also been reported to impair complement regulation in aHUS. More recently, gain of function mutations in the complement components C3 and Factor B have been seen. This review focuses on the genetic causes of aHUS, their functional consequences, and clinical effect.
Figures
Fig. 1
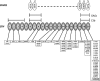
Complement regulation. The alternative complement pathway (AP) is a positive-feedback amplification loop. The AP constantly undergoes tick over but can also be primed by the classical (CP) and lectin (LP) pathways. The C3b that is formed interacts with factor B (B), which is then cleaved by factor D (D) to form the AP C3 convertase (C3bBb). This enzyme complex is attached to the target covalently via C3b while Bb is the catalytic serine protease subunit. It is stabilized by binding properdin (P). Because C3 is the substrate for this convertase, a powerful feedback loop is created. Unchecked, this will lead to activation of the terminal complement pathway with generation of the effector molecules; the anaphylatoxin C5a and the membrane attack complex (MAC). To limit damage to host cells, the AP is down-regulated by cofactor activity (CA) and decay accelerating activity (DAA). CA results in the permanent inactivation of C3b to iC3b, such that it is no longer capable of binding B and thus cannot form the AP convertase. CA requires both a cofactor protein (factor H [fH] or membrane cofactor protein [MCP]) and a protease, factor I (fI). DAA is the dissociation of the C3/C5 convertases. The decay accelerator protein, in this example decay accelerating factor (DAF), displaces the catalytic Bb from target-bound C3b. However, this C3b can bind another B and then reform the convertase
Fig. 2
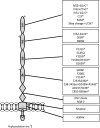
Complement factor H and complement factor H-related 1. The figure demonstrates the 20 CCP modules of factor H (bottom) and the five CCP modules of factor H-related protein 1 (top). The homology of each factor H-related protein 1 CCP to the corresponding factor H CCP is given as a percentage in the factor H-related protein 1 figure. Note the homology between CCP18 of factor H and CCP 3 of factor H-related protein 1 is given for the basic isoform. An acidic isoform differs by three amino acids [62]. The glycosaminoglycan and C3b binding sites of factor H are indicated on the diagram. Mutations in CFH reported in aHUS are listed below the figure
Fig. 3
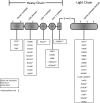
Membrane cofactor protein. The figure demonstrates clustering of mutations in the four extracellular CCP domains of membrane cofactor protein in aHUS. * denotes mutations resulting in decreased cell surface expression of membrane cofactor protein
Fig. 4
Complement factor I. The figure demonstrates the modular structure of factor I with aHUS-associated mutations below. $ denotes factor I mutants that are secreted but have been demonstrated to have decreased activity. * denotes factor I mutants that have impaired secretion
Similar articles
- Atypical hemolytic uremic syndrome.
Loirat C, Frémeaux-Bacchi V. Loirat C, et al. Orphanet J Rare Dis. 2011 Sep 8;6:60. doi: 10.1186/1750-1172-6-60. Orphanet J Rare Dis. 2011. PMID: 21902819 Free PMC article. Review. - Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype.
Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, Daina E, Fenili C, Castelletti F, Sorosina A, Piras R, Donadelli R, Maranta R, van der Meer I, Conway EM, Zipfel PF, Goodship TH, Remuzzi G. Noris M, et al. Clin J Am Soc Nephrol. 2010 Oct;5(10):1844-59. doi: 10.2215/CJN.02210310. Epub 2010 Jul 1. Clin J Am Soc Nephrol. 2010. PMID: 20595690 Free PMC article. - [Atypical hemolytic-uremic syndrome related to abnormalities within the complement system].
Frémeaux-Bacchi V, Fakhouri F, Roumenina L, Dragon-Durey MA, Loirat C. Frémeaux-Bacchi V, et al. Rev Med Interne. 2011 Apr;32(4):232-40. doi: 10.1016/j.revmed.2009.09.039. Epub 2011 Mar 3. Rev Med Interne. 2011. PMID: 21376430 French. - Atypical hemolytic uremic syndrome.
Kavanagh D, Goodship TH, Richards A. Kavanagh D, et al. Semin Nephrol. 2013 Nov;33(6):508-30. doi: 10.1016/j.semnephrol.2013.08.003. Semin Nephrol. 2013. PMID: 24161037 Free PMC article. Review. - Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype.
Bresin E, Rurali E, Caprioli J, Sanchez-Corral P, Fremeaux-Bacchi V, Rodriguez de Cordoba S, Pinto S, Goodship TH, Alberti M, Ribes D, Valoti E, Remuzzi G, Noris M; European Working Party on Complement Genetics in Renal Diseases. Bresin E, et al. J Am Soc Nephrol. 2013 Feb;24(3):475-86. doi: 10.1681/ASN.2012090884. Epub 2013 Feb 21. J Am Soc Nephrol. 2013. PMID: 23431077 Free PMC article.
Cited by
- A complicated case of atypical hemolytic uremic syndrome with frequent relapses under eculizumab.
Schalk G, Kirschfink M, Wehling C, Gastoldi S, Bergmann C, Hoppe B, Weber LT. Schalk G, et al. Pediatr Nephrol. 2015 Jun;30(6):1039-42. doi: 10.1007/s00467-015-3078-6. Epub 2015 Mar 10. Pediatr Nephrol. 2015. PMID: 25752761 - Atypical hemolytic uremic syndrome.
Loirat C, Frémeaux-Bacchi V. Loirat C, et al. Orphanet J Rare Dis. 2011 Sep 8;6:60. doi: 10.1186/1750-1172-6-60. Orphanet J Rare Dis. 2011. PMID: 21902819 Free PMC article. Review. - Utilization Pattern for Eculizumab Among Children With Hemolytic Uremic Syndrome.
Ranabothu S, Brown CC, Blaszak R, Millner R, Moore KR, Prodhan P. Ranabothu S, et al. Front Pediatr. 2021 Oct 5;9:733042. doi: 10.3389/fped.2021.733042. eCollection 2021. Front Pediatr. 2021. PMID: 34676187 Free PMC article. - Complements Spurned: Our Experience with Atypical Hemolytic Uremic Syndrome.
Nagar VS, Chaterjee R, Sood A, Sajjan B, Kaushik A, Vyahalkar SV. Nagar VS, et al. Indian J Crit Care Med. 2017 Feb;21(2):102-104. doi: 10.4103/ijccm.IJCCM_341_16. Indian J Crit Care Med. 2017. PMID: 28250608 Free PMC article. - Complement activation: an atypical presentation of an atypical syndrome.
Iardino A, Bunin V, Truong LD, Preti HA. Iardino A, et al. BMJ Case Rep. 2017 Oct 30;2017:bcr2017221798. doi: 10.1136/bcr-2017-221798. BMJ Case Rep. 2017. PMID: 29084740 Free PMC article.
References
- Fogo A, Kashgarian M. Diagnostic atlas of renal pathology. Amsterdam: Elsevier Science; 2005.
- Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–1086. - PubMed
- Ariceta G, Besbas N, Johnson S, Karpman D, Landau D, Licht C, Loirat C, Pecoraro C, Taylor CM, Kar N, Vandewalle J, Zimmerhackl LB. Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatr Nephrol. 2009;24:687–696. doi: 10.1007/s00467-008-0964-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous