Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR - PubMed (original) (raw)
. 2010 Sep 9;116(10):1685-97.
doi: 10.1182/blood-2010-01-264960. Epub 2010 Jun 7.
Anne-Laure Flamar, Yanying Cao, Jean-Philippe Blanck, Maochang Liu, Amy O'Bar, Olivier Agouna-Deciat, Peter Klucar, Luann Thompson-Snipes, Sandra Zurawski, Yoram Reiter, A Karolina Palucka, Gerard Zurawski, Jacques Banchereau
Affiliations
- PMID: 20530286
- PMCID: PMC2947393
- DOI: 10.1182/blood-2010-01-264960
Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR
Eynav Klechevsky et al. Blood. 2010.
Abstract
We evaluated human CD8(+) T-cell responses generated by targeting antigens to dendritic cells (DCs) through various lectin receptors. We found the immunoreceptor tyrosine-based inhibitory motif-containing DC immunoreceptor (DCIR) to mediate potent cross-presentation. A single exposure to a low dose of anti-DCIR-antigen conjugate initiated antigen-specific CD8(+) T-cell immunity by all human DC subsets including ex vivo-generated DCs, skin-isolated Langerhans cells, and blood myeloid DCs and plasmacytoid DCs. The delivery of influenza matrix protein (FluMP) through DCIR resulted in expansion of FluMP-specific memory CD8(+) T cells. Enhanced specific CD8(+) T-cell responses were observed when an antigen was delivered to the DCs via DCIR, compared with those induced by a free antigen, or antigen conjugated to a control monoclonal antibody or delivered via DC-SIGN, another lectin receptor. DCIR targeting also induced primary CD8(+) T-cell responses against self (MART-1) and viral (HIV gag) antigens. Addition of Toll-like receptor (TLR) 7/8 agonist enhanced DCIR-mediated cross-presentation as well as cross-priming, particularly when combined with a CD40 signal. TLR7/8 activation was associated with increased expansion of the primed CD8(+) T cells, high production of interferon-γ and tumor necrosis factor-α, and reduced levels of type 2-associated cytokines. Thus, antigen targeting via the human DCIR receptor allows activation of specific CD8(+) T-cell immunity.
Figures
Figure 1
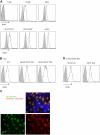
Cellular distribution of DCIR. (A) Flow cytometry analysis of DCIR expression on peripheral blood mononuclear cells. Circulating mononuclear cells were stained with 10 μg/mL anti-DCIR mAb followed by phycoerythrin-conjugated goat anti–mouse IgG. Cells were incubated with anti-CD19, anti-CD4, anti-CD8 (for lymphocytes), anti-CD16, anti-CD56 (for NK cells) not shown, anti-CD14 mAb (for monocytes), or with anti-CD11c, anti–HLA-DR, and anti-CD123 mAb (for pDCs or mDCs) and analyzed by flow cytometry. Data are representative of 3 independent experiments performed on 3 different donors. (B) Expression analysis of DCIR by flow cytometry on skin-derived DC subsets: epidermal LCs, dermal CD1a+ DCs, and dermal CD14+ DCs. (C) Human epidermal sheets, stained with anti-DCIR and analyzed by fluorescence microscopy, revealed the expression of DCIR on HLA-DR+ LCs. Image was captured using an Olympus BX51 microscope with Planapo 40×/0.95 dry objective, Photometrics Coolsnap HQ camera, and Metamorph software Version 6.2r6. Channel separation was done in Adobe Photoshop CS. (D) Expression analysis of DCIR by flow cytometry on CD34+-derived DC subsets CD1a+ LCs and CD14+ DCs.
Figure 2
Engineering and characterization of targeted proteins into DCIR mAb. (A) Five constructs are shown. (I) Diagram of mouse IgG1 cross-linked to the target antigen FluMP. (II-III) Diagram of chimeric mAbs (IgG4).doc conjugated to coh.antigen (FluMP [II] or MART-1 [III]). (IV-V) Diagram of chimeric fusion mAb IgG4-antigen (MART-1 [IV] or HIV gag p24 [V]). (B) Staining of HLA-A201–FluMP complexes on CD34+-derived DCs unpulsed (control DCs, gray histogram), or pulsed with 50nM DCIR-targeted FluMP. Cells were activated with 5 μg/mL anti-CD40 mAb (12E12, Baylor Research Institute; BIIR) and stained after 24 hours with phycoerythrin-labeled tetramerized anti–HLA-A201–FluMP Fab (M1D12). (C) Cross-presentation of FluMP to CD8+ T cells by autologous HLA-A201+CD34+–derived LCs that were cultured with 8nM (top panel) or 0.8nM (bottom panel) of anti–DCIR.doc-coh.FluMP or IgG4.doc-coh.FluMP conjugate mAbs. Dot plots show the proportions of HLA-A201–FluMP(58-66) peptide tetramer-positive CD8+ T cells after 10 days. (D) Proportions of HLA-A201–FluMP(58-66) tetramer-positive CD8+ T cells induced by DCs that were pulsed for 18 hours with 8nM anti–DCIR.doc-coh.FluMP or control IgG2a.doc-coh.FluMP conjugate mAbs, washed and cultured with autologous CD8+ T cells for 10 days. Graphs show the proportions of HLA-A201–FluMP(58-66) tetramer-positive CD8+ T cells, mean ± SD; n = 3.
Figure 3
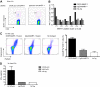
DCIR allows cross-presentation of proteins by LCs. (A) Skin-derived LCs from an HLA-A201+ donor were targeted with 8nM each of anti-DC.doc-coh.FluMP, IgG4.doc-coh.FluMP conjugate mAbs, or free FluMP matured with CD40L, and cocultured with autologous CD8+ T cells. Ten days later, CD8+ T-cell expansion was evaluated by specific HLA-A201–FluMP(58-66) tetramer staining. Data are representative of 2 independent experiments performed with cells from 2 different donors. (B) IFN-γ levels as measured by Luminex in the culture supernatant of CD8+ T cells expanded for 10 days by autologous skin LCs targeted with anti–DCIR.doc-coh.FluMP or IgG4.doc-coh.FluMP conjugate mAbs. Graph represents mean ± SD; n = 3.
Figure 4
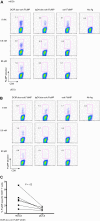
DCIR is a global target for all blood DC subsets. (A) Blood-derived mDCs from an HLA-A201+ donor are targeted with 8nM, 0.8nM, or 80pM each anti–DCIR.doc-coh.FluMP (clone 24A5), IgG4.doc-coh.FluMP conjugate mAbs, or free coh.FluMP, matured with CD40L and cocultured with autologous CD8+ T cells. Ten days later, CD8+ T-cell expansion was evaluated by specific HLA-A201–FluMP(58-66) tetramer staining. Data are representative of 3 independent experiments. (B) Blood-derived pDCs from an HLA-A201+ donor were targeted with 8nM, 0.8nM, or 80pM each anti–DCIR.doc-coh.FluMP (clone 24A5), IgG4.doc-coh.FluMP, or free coh.FluMP, matured with CD40L and cocultured with autologous CD8+ T cells. Ten days later, T-cell expansion was evaluated by specific HLA-A201–FluMP(58-66) tetramer staining. Data are representative of 3 independent experiments. (C) Percentage of FluMP-specific CD8+ T cells induced by 8nM DCIR.doc-coh.FluMP complex mAb-targeted mDCs or pDCs. Graph represents results of 3 independent experiments using 2 different clones of DCIR mAb. P = .02.
Figure 5
Cross-priming of Mart-1 and HIV gag p24 protein by anti-DCIR fusion mAb. (A) Skin-derived LCs from an HLA-A201+ donor were purified and cultured for 10 days with autologous purified T cells in the presence of 30nM anti–DCIR.doc-coh.MART-1 or IgG4.doc-coh.MART-1 conjugate mAbs. DCs were activated with CD40L. MART-1–specific CD8+ T-cell expansion was measured with a specific HLA-A201-MART-1(26-35) tetramer. (B) Anti–DCIR-MART-1 or IgG4-MART-1 (25nM) fusion proteins were used to target monocyte-derived IFN-α DCs. DCs were activated with CD40L and cultured with naive autologous CD8+ T cells. After 10 days, cells were restimulated for 24 hours with fresh DCs loaded with peptides derived from MART-1 protein or with unloaded DCs as a control. Plot shows the percentage of primed CD8+ T cells coexpressing IFN-γ and CD107a in response to a specific MART-1 peptide cluster. (C) CD34+-derived LCs were targeted with DCIR-MART-1 or control IgG4-MART-1 fusion proteins and cultured with naive CD8+ T cells for 9 days. Graph represents the percentage of cells coexpressing Granzyme B and perforin as analyzed at the end of the culture by flow cytometry. Values in the graph are the average of triplicates ± SD. Data are representative of 2 independent experiments. (D) Anti–DCIR-p24 or control IgG4-p24 (25nM) fusion proteins were used to target CD34+-derived LCs. DCs were activated with CD40L and cultured with naive autologous CD8+ T cells. After 2 consecutive stimulations, the proliferated cells were sorted and restimulated for 24 hours with fresh LCs and HIV gag p24 protein to evaluate IFN-γ secretion by Luminex. Cells with no protein served as a control. Values are average of duplicates. Data are representative of 2 independent experiments.
Figure 6
TLR7/8-signaling enhances DCIR-mediated secondary CD8+ T-cell response by mDCs. (A) Blood-derived mDCs from an HLA-A201+ donor were targeted with 12nM, 2nM, or 200pM of anti–DCIR.doc-coh.FluMP complex mAb, activated with either TLR3, TLR4, or TLR7/8 agonists (poly I:C, LPS, or CL075) and cocultured with autologous CD8+ T cells for 10 days. Graph represents the percentage of FluMP-specific CD8+ T cells measured with a specific HLA-A201–FluMP(58-66) tetramer for each amount of anti–DCIR.doc-coh.FluMP complex mAb and with each DC-activator tested. DCs with no activation were used as a control: no activation (—), TLR7/8 (♦), TLR3 (*), and TLR4 (○) agonists; CL075, poly I:C, and LPS, respectively. Data are representative of 4 independent experiments with 4 different donors. The graph represents mean ± SD; n = 3. (B) Blood-derived mDCs from an HLA-A201+ donor were targeted with 8nM anti–DCIR.doc-coh.FluMP or IgG4.doc-coh.FluMP complex mAb, activated with TLR7/8, TLR3, and TLR4 agonists (CL075, poly I:C, and LPS, respectively) and cocultured with autologous CD8+ T cells for 10 days. Graph represents the percentage of FluMP-specific CD8+ T cells as measured with a specific HLA-A201–FluMP(58-66) tetramer. Conditions indicated in the graph are as follows: no activation, CL075 1μg/mL; poly I:C, 10 μg/mL; and LPS, 50 ng/mL. The graph represents mean ± SD; n = 3. (C) Same experiment as in panel B. Graph represents the mean percentage of FluMP-specific CD8+ T cells as measured with a specific HLA-A201–FluMP(58-66) tetramer. Conditions indicated in the graph are as follows: no activation; CL075-0.2μg/mL and 2 μg/mL; poly I:C, 5 μg/mL and 25 μg/mL; LPS, 10 ng/mL and 100 ng/mL.
Figure 7
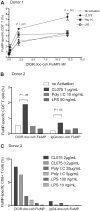
TLR7/8-signaling enhances DCIR-mediated primary CD8+ T-cell response by mDCs. (A) Blood-derived mDCs from an HLA-A201+ donor were targeted with 17nM of anti–DCIR-MART-1 or a control IgG4-MART-1 fusion proteins, activated with CD40L (100 ng/mL), CL075 (1 μg/mL), poly I:C (5 μg/mL), or LPS (50 ng/mL) and cocultured with autologous naive CD8+ T cells for 10 days. The expansion of MART-1–specific CD8+ T cells was measured with a specific HLA-A201-MART-1(26-35) tetramer. Data are of 2 independent experiments with 2 different donors. (B) Blood-derived mDCs from an HLA-A201+ donor were targeted with 30nM of anti–DCIR-MART-1 fusion protein or anti–DCIR-p24, activated with either CD40L or TLR7/8 agonists, and cocultured with autologous naive CD8+ T cells for 10 days. (Top panel) The proportions of HLA-A201-MART-1(26-35) peptide tetramer-positive CD8+ T cells expanded by purified blood mDCs cultured with anti–DCIR-MART-1 fusion protein and activated with either CD40L or TLR7/8 agonist. (Bottom panel) The proportions of HLA-A201-HIV gag p24(151-159) peptide tetramer-positive CD8+ T cells expanded by purified blood mDCs targeted with anti–DCIR-p24 fusion protein and activated with either CD40L or TLR7/8 agonist. Data are of 2 independent experiments with 2 different donors. (C) The expression of intracellular effector molecules Granzyme B and perforin was assessed by flow cytometry on CD8+ T cells primed by IFN-α DCs targeted with 10nM of anti–DCIR-MART-1 or IgG4-MART-1 fusion proteins and activated with CD40L, CL075, or a combination of CD40L and CL075. The expression on the antigen specific MART-1(26-35)-positive cells was analyzed by co staining with the corresponding HLA-A201-tetramer. Data are representative of 2 independent experiments. (D) The frequency of MART-1–specific CD8+ T cells, as measured with a specific HLA-A201-MART-1(26-35) tetramer, after expansion with anti–DCIR-MART-1–targeted DCs that were activated with CD40L, TLR7/8 ligand, or a combination of CD40L and TLR7/8 ligand. IgG4-MART-1 fusion protein or no antigen conditions served as controls. Each dot represents a single experiment. (E top panel) IFN-α DCs were targeted with 17nM of anti–DCIR-MART-1 or a control IgG4-MART-1 fusion proteins, activated with CD40L (100 ng/mL), CL075 (1 μg/mL), poly I:C (10 μg/mL), or LPS (50 ng/mL) and cocultured with autologous naive CD8+ T cells. Ten days later, cells were restimulated with fresh DCs that were loaded with 15mer overlapping peptides derived from the MART-1 protein. Plots show the level of intracytoplasmic IFN-γ by CD8+ T cells after 5-hour stimulation in the presence of monensin. Data are representative of 3 independent experiments. (Bottom panel) Anti–DCIR-p24 or a control IgG4-p24 fusion proteins were used as a model antigen. (F) IFN-α DCs were targeted with 113nM anti–DCIR-MART-1 fusion protein activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL) and cocultured with autologous naive CD8+ T cells. Ten days later, cells were restimulated with fresh DCs that were loaded with 15mer overlapping peptides derived from the MART-1 protein. The levels of IL-4, IL-5, IL-13, IFN-γ, TNF-α, and IL-12p40 were measured by Luminex in the culture supernatant after 24 hours. The graph represents mean ± SD; n = 3. (G) IFN-α DCs were targeted with 10nM anti–DCIR-MART-1 (▴) or a control IgG4-MART-1 ( ) fusion proteins activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL), or a combination of CD40L and CL075 and cocultured with autologous naive CD8+ T cells. Coculture in the absence of an antigen served as an additional control (□). Ten days later, cells were restimulated with fresh IFN-α DCs that were loaded with MART-1 fusion protein and analyzed by flow cytometry for their intracellular cytokine production. Graphs show the frequency of IFN-γ (left panel) and IFN-γ+TNF-α+ (right panel) producing CD8+ T cells primed by DCIR-targeted, or control IFN-α DCs after 5-hour restimulation in the presence of monensin and 0.25 μg/mL of anti-CD28/CD49d mAb (n = 3).
) fusion proteins activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL), or a combination of CD40L and CL075 and cocultured with autologous naive CD8+ T cells. Coculture in the absence of an antigen served as an additional control (□). Ten days later, cells were restimulated with fresh IFN-α DCs that were loaded with MART-1 fusion protein and analyzed by flow cytometry for their intracellular cytokine production. Graphs show the frequency of IFN-γ (left panel) and IFN-γ+TNF-α+ (right panel) producing CD8+ T cells primed by DCIR-targeted, or control IFN-α DCs after 5-hour restimulation in the presence of monensin and 0.25 μg/mL of anti-CD28/CD49d mAb (n = 3).
Figure 7
TLR7/8-signaling enhances DCIR-mediated primary CD8+ T-cell response by mDCs. (A) Blood-derived mDCs from an HLA-A201+ donor were targeted with 17nM of anti–DCIR-MART-1 or a control IgG4-MART-1 fusion proteins, activated with CD40L (100 ng/mL), CL075 (1 μg/mL), poly I:C (5 μg/mL), or LPS (50 ng/mL) and cocultured with autologous naive CD8+ T cells for 10 days. The expansion of MART-1–specific CD8+ T cells was measured with a specific HLA-A201-MART-1(26-35) tetramer. Data are of 2 independent experiments with 2 different donors. (B) Blood-derived mDCs from an HLA-A201+ donor were targeted with 30nM of anti–DCIR-MART-1 fusion protein or anti–DCIR-p24, activated with either CD40L or TLR7/8 agonists, and cocultured with autologous naive CD8+ T cells for 10 days. (Top panel) The proportions of HLA-A201-MART-1(26-35) peptide tetramer-positive CD8+ T cells expanded by purified blood mDCs cultured with anti–DCIR-MART-1 fusion protein and activated with either CD40L or TLR7/8 agonist. (Bottom panel) The proportions of HLA-A201-HIV gag p24(151-159) peptide tetramer-positive CD8+ T cells expanded by purified blood mDCs targeted with anti–DCIR-p24 fusion protein and activated with either CD40L or TLR7/8 agonist. Data are of 2 independent experiments with 2 different donors. (C) The expression of intracellular effector molecules Granzyme B and perforin was assessed by flow cytometry on CD8+ T cells primed by IFN-α DCs targeted with 10nM of anti–DCIR-MART-1 or IgG4-MART-1 fusion proteins and activated with CD40L, CL075, or a combination of CD40L and CL075. The expression on the antigen specific MART-1(26-35)-positive cells was analyzed by co staining with the corresponding HLA-A201-tetramer. Data are representative of 2 independent experiments. (D) The frequency of MART-1–specific CD8+ T cells, as measured with a specific HLA-A201-MART-1(26-35) tetramer, after expansion with anti–DCIR-MART-1–targeted DCs that were activated with CD40L, TLR7/8 ligand, or a combination of CD40L and TLR7/8 ligand. IgG4-MART-1 fusion protein or no antigen conditions served as controls. Each dot represents a single experiment. (E top panel) IFN-α DCs were targeted with 17nM of anti–DCIR-MART-1 or a control IgG4-MART-1 fusion proteins, activated with CD40L (100 ng/mL), CL075 (1 μg/mL), poly I:C (10 μg/mL), or LPS (50 ng/mL) and cocultured with autologous naive CD8+ T cells. Ten days later, cells were restimulated with fresh DCs that were loaded with 15mer overlapping peptides derived from the MART-1 protein. Plots show the level of intracytoplasmic IFN-γ by CD8+ T cells after 5-hour stimulation in the presence of monensin. Data are representative of 3 independent experiments. (Bottom panel) Anti–DCIR-p24 or a control IgG4-p24 fusion proteins were used as a model antigen. (F) IFN-α DCs were targeted with 113nM anti–DCIR-MART-1 fusion protein activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL) and cocultured with autologous naive CD8+ T cells. Ten days later, cells were restimulated with fresh DCs that were loaded with 15mer overlapping peptides derived from the MART-1 protein. The levels of IL-4, IL-5, IL-13, IFN-γ, TNF-α, and IL-12p40 were measured by Luminex in the culture supernatant after 24 hours. The graph represents mean ± SD; n = 3. (G) IFN-α DCs were targeted with 10nM anti–DCIR-MART-1 (▴) or a control IgG4-MART-1 ( ) fusion proteins activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL), or a combination of CD40L and CL075 and cocultured with autologous naive CD8+ T cells. Coculture in the absence of an antigen served as an additional control (□). Ten days later, cells were restimulated with fresh IFN-α DCs that were loaded with MART-1 fusion protein and analyzed by flow cytometry for their intracellular cytokine production. Graphs show the frequency of IFN-γ (left panel) and IFN-γ+TNF-α+ (right panel) producing CD8+ T cells primed by DCIR-targeted, or control IFN-α DCs after 5-hour restimulation in the presence of monensin and 0.25 μg/mL of anti-CD28/CD49d mAb (n = 3).
) fusion proteins activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL), or a combination of CD40L and CL075 and cocultured with autologous naive CD8+ T cells. Coculture in the absence of an antigen served as an additional control (□). Ten days later, cells were restimulated with fresh IFN-α DCs that were loaded with MART-1 fusion protein and analyzed by flow cytometry for their intracellular cytokine production. Graphs show the frequency of IFN-γ (left panel) and IFN-γ+TNF-α+ (right panel) producing CD8+ T cells primed by DCIR-targeted, or control IFN-α DCs after 5-hour restimulation in the presence of monensin and 0.25 μg/mL of anti-CD28/CD49d mAb (n = 3).
Figure 7
TLR7/8-signaling enhances DCIR-mediated primary CD8+ T-cell response by mDCs. (A) Blood-derived mDCs from an HLA-A201+ donor were targeted with 17nM of anti–DCIR-MART-1 or a control IgG4-MART-1 fusion proteins, activated with CD40L (100 ng/mL), CL075 (1 μg/mL), poly I:C (5 μg/mL), or LPS (50 ng/mL) and cocultured with autologous naive CD8+ T cells for 10 days. The expansion of MART-1–specific CD8+ T cells was measured with a specific HLA-A201-MART-1(26-35) tetramer. Data are of 2 independent experiments with 2 different donors. (B) Blood-derived mDCs from an HLA-A201+ donor were targeted with 30nM of anti–DCIR-MART-1 fusion protein or anti–DCIR-p24, activated with either CD40L or TLR7/8 agonists, and cocultured with autologous naive CD8+ T cells for 10 days. (Top panel) The proportions of HLA-A201-MART-1(26-35) peptide tetramer-positive CD8+ T cells expanded by purified blood mDCs cultured with anti–DCIR-MART-1 fusion protein and activated with either CD40L or TLR7/8 agonist. (Bottom panel) The proportions of HLA-A201-HIV gag p24(151-159) peptide tetramer-positive CD8+ T cells expanded by purified blood mDCs targeted with anti–DCIR-p24 fusion protein and activated with either CD40L or TLR7/8 agonist. Data are of 2 independent experiments with 2 different donors. (C) The expression of intracellular effector molecules Granzyme B and perforin was assessed by flow cytometry on CD8+ T cells primed by IFN-α DCs targeted with 10nM of anti–DCIR-MART-1 or IgG4-MART-1 fusion proteins and activated with CD40L, CL075, or a combination of CD40L and CL075. The expression on the antigen specific MART-1(26-35)-positive cells was analyzed by co staining with the corresponding HLA-A201-tetramer. Data are representative of 2 independent experiments. (D) The frequency of MART-1–specific CD8+ T cells, as measured with a specific HLA-A201-MART-1(26-35) tetramer, after expansion with anti–DCIR-MART-1–targeted DCs that were activated with CD40L, TLR7/8 ligand, or a combination of CD40L and TLR7/8 ligand. IgG4-MART-1 fusion protein or no antigen conditions served as controls. Each dot represents a single experiment. (E top panel) IFN-α DCs were targeted with 17nM of anti–DCIR-MART-1 or a control IgG4-MART-1 fusion proteins, activated with CD40L (100 ng/mL), CL075 (1 μg/mL), poly I:C (10 μg/mL), or LPS (50 ng/mL) and cocultured with autologous naive CD8+ T cells. Ten days later, cells were restimulated with fresh DCs that were loaded with 15mer overlapping peptides derived from the MART-1 protein. Plots show the level of intracytoplasmic IFN-γ by CD8+ T cells after 5-hour stimulation in the presence of monensin. Data are representative of 3 independent experiments. (Bottom panel) Anti–DCIR-p24 or a control IgG4-p24 fusion proteins were used as a model antigen. (F) IFN-α DCs were targeted with 113nM anti–DCIR-MART-1 fusion protein activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL) and cocultured with autologous naive CD8+ T cells. Ten days later, cells were restimulated with fresh DCs that were loaded with 15mer overlapping peptides derived from the MART-1 protein. The levels of IL-4, IL-5, IL-13, IFN-γ, TNF-α, and IL-12p40 were measured by Luminex in the culture supernatant after 24 hours. The graph represents mean ± SD; n = 3. (G) IFN-α DCs were targeted with 10nM anti–DCIR-MART-1 (▴) or a control IgG4-MART-1 ( ) fusion proteins activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL), or a combination of CD40L and CL075 and cocultured with autologous naive CD8+ T cells. Coculture in the absence of an antigen served as an additional control (□). Ten days later, cells were restimulated with fresh IFN-α DCs that were loaded with MART-1 fusion protein and analyzed by flow cytometry for their intracellular cytokine production. Graphs show the frequency of IFN-γ (left panel) and IFN-γ+TNF-α+ (right panel) producing CD8+ T cells primed by DCIR-targeted, or control IFN-α DCs after 5-hour restimulation in the presence of monensin and 0.25 μg/mL of anti-CD28/CD49d mAb (n = 3).
) fusion proteins activated with either CD40L (100 ng/mL) or CL075 (1 μg/mL), or a combination of CD40L and CL075 and cocultured with autologous naive CD8+ T cells. Coculture in the absence of an antigen served as an additional control (□). Ten days later, cells were restimulated with fresh IFN-α DCs that were loaded with MART-1 fusion protein and analyzed by flow cytometry for their intracellular cytokine production. Graphs show the frequency of IFN-γ (left panel) and IFN-γ+TNF-α+ (right panel) producing CD8+ T cells primed by DCIR-targeted, or control IFN-α DCs after 5-hour restimulation in the presence of monensin and 0.25 μg/mL of anti-CD28/CD49d mAb (n = 3).
Similar articles
- Monocyte-derived dendritic cells loaded with a mixture of apoptotic/necrotic melanoma cells efficiently cross-present gp100 and MART-1 antigens to specific CD8(+) T lymphocytes.
von Euw EM, Barrio MM, Furman D, Bianchini M, Levy EM, Yee C, Li Y, Wainstok R, Mordoh J. von Euw EM, et al. J Transl Med. 2007 Apr 20;5:19. doi: 10.1186/1479-5876-5-19. J Transl Med. 2007. PMID: 17448240 Free PMC article. - Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production.
Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ, Adema GJ. Meyer-Wentrup F, et al. Blood. 2008 Apr 15;111(8):4245-53. doi: 10.1182/blood-2007-03-081398. Epub 2008 Feb 7. Blood. 2008. PMID: 18258799 - Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A.
Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, Huang Y, Rodriguez A, Clausen BE, Park CG, Trumpfheller C, Steinman RM. Idoyaga J, et al. Proc Natl Acad Sci U S A. 2011 Feb 8;108(6):2384-9. doi: 10.1073/pnas.1019547108. Epub 2011 Jan 24. Proc Natl Acad Sci U S A. 2011. PMID: 21262813 Free PMC article. - Signaling and immune regulatory role of the dendritic cell immunoreceptor (DCIR) family lectins: DCIR, DCAR, dectin-2 and BDCA-2.
Kanazawa N, Tashiro K, Miyachi Y. Kanazawa N, et al. Immunobiology. 2004;209(1-2):179-90. doi: 10.1016/j.imbio.2004.03.004. Immunobiology. 2004. PMID: 15481152 Review. - Dendritic cell immunoreceptors: C-type lectin receptors for pattern-recognition and signaling on antigen-presenting cells.
Kanazawa N. Kanazawa N. J Dermatol Sci. 2007 Feb;45(2):77-86. doi: 10.1016/j.jdermsci.2006.09.001. Epub 2006 Oct 13. J Dermatol Sci. 2007. PMID: 17046204 Review.
Cited by
- The Dectin-1 and Dectin-2 clusters: C-type lectin receptors with fundamental roles in immunity.
Malamud M, Brown GD. Malamud M, et al. EMBO Rep. 2024 Oct 31. doi: 10.1038/s44319-024-00296-2. Online ahead of print. EMBO Rep. 2024. PMID: 39482490 Review. - Straight to the point: targeted mRNA-delivery to immune cells for improved vaccine design.
Clemente B, Denis M, Silveira CP, Schiavetti F, Brazzoli M, Stranges D. Clemente B, et al. Front Immunol. 2023 Nov 27;14:1294929. doi: 10.3389/fimmu.2023.1294929. eCollection 2023. Front Immunol. 2023. PMID: 38090568 Free PMC article. Review. - Oct4 and Hypoxia Dual-Regulated Oncolytic Adenovirus Armed with shRNA-Targeting Dendritic Cell Immunoreceptor Exerts Potent Antitumor Activity against Bladder Cancer.
Hu CY, Hung CF, Chen PC, Hsu JY, Wang CT, Lai MD, Tsai YS, Shiau AL, Shieh GS, Wu CL. Hu CY, et al. Biomedicines. 2023 Sep 22;11(10):2598. doi: 10.3390/biomedicines11102598. Biomedicines. 2023. PMID: 37892972 Free PMC article. - Current perspective on biological properties of plasmacytoid dendritic cells and dysfunction in gut.
Guo X, He C, Xin S, Gao H, Wang B, Liu X, Zhang S, Gong F, Yu X, Pan L, Sun F, Xu J. Guo X, et al. Immun Inflamm Dis. 2023 Sep;11(9):e1005. doi: 10.1002/iid3.1005. Immun Inflamm Dis. 2023. PMID: 37773693 Free PMC article. Review. - Controlling Antigen Fate in Therapeutic Cancer Vaccines by Targeting Dendritic Cell Receptors.
Wijfjes Z, van Dalen FJ, Le Gall CM, Verdoes M. Wijfjes Z, et al. Mol Pharm. 2023 Oct 2;20(10):4826-4847. doi: 10.1021/acs.molpharmaceut.3c00330. Epub 2023 Sep 18. Mol Pharm. 2023. PMID: 37721387 Free PMC article. Review.
References
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. - PubMed
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. - PubMed
- Ueno H, Klechevsky E, Morita R, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. - PubMed
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7(1):19–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P0-1 CA84512/CA/NCI NIH HHS/United States
- R0-1 CA85540/CA/NCI NIH HHS/United States
- R01 CA085540/CA/NCI NIH HHS/United States
- P01 CA084512/CA/NCI NIH HHS/United States
- U19 AI057234/AI/NIAID NIH HHS/United States
- U-19 AI-57234/AI/NIAID NIH HHS/United States
- R01 CA078846/CA/NCI NIH HHS/United States
- R0-1 CA78846/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous