Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation - PubMed (original) (raw)
Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation
Indira Jutooru et al. J Biol Chem. 2010.
Abstract
Curcumin activates diverse anticancer activities that lead to inhibition of cancer cell and tumor growth, induction of apoptosis, and antiangiogenic responses. In this study, we observed that curcumin inhibits Panc28 and L3.6pL pancreatic cancer cell and tumor growth in nude mice bearing L3.6pL cells as xenografts. In addition, curcumin decreased expression of p50 and p65 proteins and NFkappaB-dependent transactivation and also decreased Sp1, Sp3, and Sp4 transcription factors that are overexpressed in pancreatic cancer cells. Because both Sp transcription factors and NFkappaB regulate several common genes such as cyclin D1, survivin, and vascular endothelial growth factor that contribute to the cancer phenotype, we also investigated interactions between Sp and NFkappaB transcription factors. Results of Sp1, Sp3, and Sp4 knockdown by RNA interference demonstrate that both p50 and p65 are Sp-regulated genes and that inhibition of constitutive or tumor necrosis factor-induced NFkappaB by curcumin is dependent on down-regulation of Sp1, Sp3, and Sp4 proteins by this compound. Curcumin also decreased mitochondrial membrane potential and induced reactive oxygen species in pancreatic cancer cells, and this pathway is required for down-regulation of Sp proteins in these cells, demonstrating that the mitochondriotoxic effects of curcumin are important for its anticancer activities.
Figures
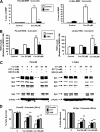
FIGURE 1.
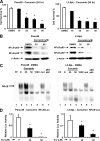
Curcumin inhibits pancreatic cancer cell growth, decreases expression of p65, p50 proteins, NFκB-DNA binding, and transactivation of the NFκB promoter. A, inhibition of Panc28 and L3.6pL cell growth. Cells were treated with DMSO (solvent control) or 10, 25, 35, or 50 μmol/liter of curcumin, and effects on cell growth were determined after treatment for 24 h as described under “Experimental Procedures.” B, effects of curcumin on p65 and p50 subunits of NFκB in Panc28 and L3.6pL cells. Cells were treated with DMSO (0), 35 or 50 μmol/liter of curcumin for 24 h, and p65 and p50 protein levels in nuclear extracts were determined as described under “Experimental Procedures.” β-Actin served as loading control. C, gel mobility shift assay. Panc28 and L3.6pL cells were treated with DMSO or 25 or 50 μmol/liter of curcumin for 24 h, and nuclear lysates were incubated with 32P-labeled GC-rich oligonucleotide alone or in the presence of other factors. Retarded bands were analyzed by electrophoretic mobility shift assay as described under “Experimental Procedures.” D, decrease in transactivation of NFκB promoter. Panc28 and L3.6pL cells were transfected with the NFκB-luc construct, then treated with DMSO or 25 and 50 μ
m
curcumin, and luciferase activity was determined as described under “Experimental Procedures.” Results are expressed as mean ± S.E. for three replicate determinations for each treatment group, and significant (p < 0.05) compared with solvent (DMSO) control indicated by an asterisk. EMSA, electrophoretic mobility shift assay.
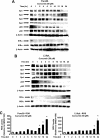
FIGURE 2.
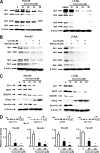
Curcumin activates proteosome-independent down-regulation of Sp proteins, decreases cell growth, angiogenic and apoptotic proteins, and their promoters. A, decreased Sp proteins. Panc28 and L3.6pL cells were treated with DMSO or 10, 25, 35, and 50 μmol/liter of curcumin for 24 h and whole cell lysates were analyzed by Western blot analysis as described under “Experimental Procedures.” B, curcumin causes proteasome-independent Sp degradation. Cells were treated with DMSO or 50 μmol/liter of curcumin in the presence or absence of proteasome inhibitor MG132, and the effects on Sp protein degradation were determined after treatment for 24 h by Western blot as described under “Experimental Procedures.” C, curcumin decreases expression of Sp-dependent gene products. Panc28 and L3.6pL cells were treated with DMSO or 10, 25, 35, or 50 μmol/liter of curcumin for 24 h, and whole cell lysates were analyzed by Western blot analysis as described under “Experimental Procedures.” β-Actin served as a loading control. D, curcumin decreases transactivation in cells transfected with Sp1, Sp3, VEGF, and survivin promoter constructs. Cells were treated with DMSO (solvent control) or 25 or 40 μmol/liter of curcumin, and the effects on transactivation of promoters were determined after treatment for 24 h as described under “Experimental Procedures.” Results are expressed as mean ± S.E. for three replicate determinations for each treatment group, and significant (p < 0.05) decreases in luciferase activity compared with the solvent (DMSO) control are indicated (*).
FIGURE 3.
Sp and NFκB knockdown and effects on NFκB subunits, angiogenic and survival proteins. Sp (A) and NFκB (B) knockdown by RNA interference. Panc28 and L3.6pL were transfected with iSp (A) or ip65/p50 (B), and effects on Sp proteins, and p65 and p50 subunits of NFκB were determined by Western blot analysis as described under “Experimental Procedures.” C, effects of Sp and NFκB knockdown on expression of CD1, VEGF, and survivin proteins. Protein lysates from Panc28 and L3.6pL cells transfected with iSp or ip65/p50 were analyzed for CD1, VEGF, and survivin proteins by Western blot analysis as described under “Experimental Procedures.” D, effects of Sp knockdown on p65, p50, cell proliferation, and PARP cleavage. Cells were transfected with various oligonucleotides, and cell numbers were counted or cell lysates were analyzed by Western blots as described under “Experimental Procedures.” E, effects of iSp on transactivation of NFκB promoter. Cells were transfected iSp and NFκB-luc, and luciferase activity was estimated as described under “Experimental Procedures.” β-Actin and Lamin served as a loading control and similar results were observed in duplicate experiments (A–C). Cell numbers (D) or luciferase activity (E) in transfection experiments were expressed as mean ± S.E. for 3 replicate experiments and significant (p < 0.05) decreases are indicated (*).
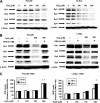
FIGURE 4.
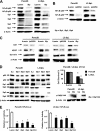
Role of Sp proteins in curcumin-dependent inhibition of TNFα inducible responses in L3.6pL pancreatic cancer cells. A, curcumin decreases TNFα-induced expression of p65 and p50 proteins. Cells were treated with TNFα in the presence or absence of 50 μ
m
curcumin, and nuclear lysates were examined for expression of p65 and p50 proteins by Western blots as described under “Experimental Procedures.” B, curcumin decreased TNFα-induced NFκB oligonucleotide-protein binding. L3.6pL cells were treated with DMSO or 50 μmol/liter of curcumin in the presence or absence of TNFα for 24 h, and nuclear extracts were incubated with 32P-labeled NFκB oligonucleotide alone or in the presence of other factors. Retarded bands were analyzed by electrophoretic mobility shift assay as described under “Experimental Procedures.” Effects of curcumin (C) and iSp (D) on Sp/NFκB-dependent protein expression are shown. L3.6pL cells were treated with 50 μ
m
curcumin (C) or transfected with iSp (D) in the presence or absence of TNFα, and whole cell and nuclear lysates were analyzed for Sp1, Sp3, Sp4, p65, p50, CD1, COX-2, and VEGF proteins by Western blot analysis as described under “Experimental Procedures.” The gels were typical of results of at least two replicate determinations per treatment group. E, effects of curcumin on TNFα-induced responses. L3.6pL cells were treated with curcumin or TNFα alone or in combination for up to 45 min, whole cell lysates were obtained and analyzed by Western blots as described under “Experimental Procedures.”
FIGURE 5.
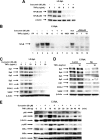
Effects of curcumin on MMP and ROS and related responses. Induction of changes in loss of MMP (A) and ROS (B) by curcumin. Panc28 and L3.6pL cells were treated with DMSO or 25 or 40 μmol/liter of curcumin for 24 h, in the presence or absence of antioxidant GSH, and mitochondrial membrane potential and ROS were determined as described under “Experimental Procedures.” ROS mediated Sp degradation (C) cell growth inhibition (D) in the presence or absence of antioxidants. Cells were treated with DMSO or 35 or 50 μmol/liter of curcumin in the presence or absence of thiol antioxidants for 24 h, and cells were then counted or the whole cell lysates were analyzed by Western blots as described under “Experimental Procedures.” β-Actin served as a loading control. Results in A, B, and D are expressed as mean ± S.E. for three replicate determinations for each treatment group, and significant (p < 0.05). Curcumin-mediated decreases (*) or increases after cotreatment with antioxidants (**) compared with the solvent (DMSO) control are indicated. GSH levels in Panc28 (4.33 μ
m
) and L3.6pL (2.64 μ
m
) cells were also determined as described under “Experimental Procedures.”
FIGURE 6.
Time course effects of curcumin on Sp1, Sp3, Sp4, p65 and p50, and ROS. Panc28 (A) and L3.6pL (B) cells were treated with DMSO (0 time) or 50 μ
m
curcumin for different times over a 24-h period and whole cell lysates were analyzed by Western blots as described under “Experimental Procedures.” C, induction of ROS. The time course induction of ROS by curcumin in Panc28 and L3.6pL cells was determined as described under “Experimental Procedures.” Results are mean ± S.E. (3 replicates/group) and significant (p < 0.05) induction of ROS is indicated (*).
FIGURE 7.
Hydrogen peroxide decreases Sp proteins and induces ROS. Effects of hydrogen peroxide alone (A) and in combination with GSH (B) are shown. Cells were treated with hydrogen peroxide or GSH alone or in combination for 24 h and whole cell lysates were analyzed by Western blots as described under “Experimental Procedures.” C, induction of ROS. Panc28 and L3.6pL cells were treated with hydrogen peroxide for 18 or 24 h and ROS was determined as described under “Experimental Procedures.” Results are mean ± S.E. (3 replicates/group) and significant (p < 0.05) induction of ROS is indicated (*).
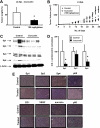
FIGURE 8.
Curcumin inhibits pancreatic cancer xenograft tumor growth. Tumor weights (A) and volume (B) are shown. Athymic nude mice bearing L3.6pL xenografts were treated with corn oil or curcumin (100 mg/kg/day), and tumor weights and volumes (mm3) were determined as described under “Experimental Procedures.” C, Western blot analysis of tumor lysates. Lysates from three mice in the treated and control groups were analyzed by Western blots as described under “Experimental Procedures.” β-Actin served as loading control and for standardizing quantitative protein determinations. D, Sp proteins levels of control animals were set at 100%. Columns, means for three separate determinations; bars, S.E.; *, significantly (p < 0.05) decreased protein levels. E, immunohistochemical staining. Tumor slides from treated and untreated animals were stained as described under “Experimental Procedures.”
Similar articles
- Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs.
Gandhy SU, Kim K, Larsen L, Rosengren RJ, Safe S. Gandhy SU, et al. BMC Cancer. 2012 Nov 30;12:564. doi: 10.1186/1471-2407-12-564. BMC Cancer. 2012. PMID: 23194063 Free PMC article. - Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation.
Abdelrahim M, Baker CH, Abbruzzese JL, Safe S. Abdelrahim M, et al. J Natl Cancer Inst. 2006 Jun 21;98(12):855-68. doi: 10.1093/jnci/djj232. J Natl Cancer Inst. 2006. PMID: 16788159 - Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors.
Chintharlapalli S, Papineni S, Lei P, Pathi S, Safe S. Chintharlapalli S, et al. BMC Cancer. 2011 Aug 24;11:371. doi: 10.1186/1471-2407-11-371. BMC Cancer. 2011. PMID: 21864401 Free PMC article. - Specificity Proteins (Sp) and Cancer.
Safe S. Safe S. Int J Mol Sci. 2023 Mar 8;24(6):5164. doi: 10.3390/ijms24065164. Int J Mol Sci. 2023. PMID: 36982239 Free PMC article. Review. - Expression of specificity protein transcription factors in pancreatic cancer and their association in prognosis and therapy.
Sankpal UT, Maliakal P, Bose D, Kayaleh O, Buchholz D, Basha R. Sankpal UT, et al. Curr Med Chem. 2012;19(22):3779-86. doi: 10.2174/092986712801661077. Curr Med Chem. 2012. PMID: 22725697 Review.
Cited by
- High concentrations of L-ascorbic acid specifically inhibit the growth of human leukemic cells via downregulation of HIF-1α transcription.
Kawada H, Kaneko M, Sawanobori M, Uno T, Matsuzawa H, Nakamura Y, Matsushita H, Ando K. Kawada H, et al. PLoS One. 2013 Apr 23;8(4):e62717. doi: 10.1371/journal.pone.0062717. Print 2013. PLoS One. 2013. PMID: 23626851 Free PMC article. - The multifaceted role of curcumin in cancer prevention and treatment.
Shanmugam MK, Rane G, Kanchi MM, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP, Sethi G. Shanmugam MK, et al. Molecules. 2015 Feb 5;20(2):2728-69. doi: 10.3390/molecules20022728. Molecules. 2015. PMID: 25665066 Free PMC article. Review. - Inhibition of rhabdomyosarcoma cell and tumor growth by targeting specificity protein (Sp) transcription factors.
Chadalapaka G, Jutooru I, Sreevalsan S, Pathi S, Kim K, Chen C, Crose L, Linardic C, Safe S. Chadalapaka G, et al. Int J Cancer. 2013 Feb 15;132(4):795-806. doi: 10.1002/ijc.27730. Epub 2012 Aug 3. Int J Cancer. 2013. PMID: 22815231 Free PMC article. - Reactive Oxygen Species (ROS)-Inducing Triterpenoid Inhibits Rhabdomyosarcoma Cell and Tumor Growth through Targeting Sp Transcription Factors.
Kasiappan R, Jutooru I, Mohankumar K, Karki K, Lacey A, Safe S. Kasiappan R, et al. Mol Cancer Res. 2019 Mar;17(3):794-805. doi: 10.1158/1541-7786.MCR-18-1071. Epub 2019 Jan 4. Mol Cancer Res. 2019. PMID: 30610105 Free PMC article. - Role of metastasis-associated lung adenocarcinoma transcript-1 (MALAT-1) in pancreatic cancer.
Cheng Y, Imanirad P, Jutooru I, Hedrick E, Jin UH, Rodrigues Hoffman A, Leal de Araujo J, Morpurgo B, Golovko A, Safe S. Cheng Y, et al. PLoS One. 2018 Feb 1;13(2):e0192264. doi: 10.1371/journal.pone.0192264. eCollection 2018. PLoS One. 2018. PMID: 29389953 Free PMC article.
References
- Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T., Thun M. J. (2008) CA Cancer J. Clin. 58, 71–96 - PubMed
- Evans D. B., Abbruzzese J. L., Willett C. G. (1997) in Cancer: Principles and Practice of Oncology (DeVita V. T., Hellman S., Rosenberg S. A. eds) pp. 1126–1161, Lippincott, Williams & Wilkins, Philadelphia
- Hruban R. H. (2001) J. Gastrointest. Surg. 5, 583–587 - PubMed
- Li D. (2001) Cancer J. 7, 259–265 - PubMed
- Gold E. B., Goldin S. B. (1998) Surg. Oncol. Clin. N. Am. 7, 67–91 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials