Immunity and immunopathology to viruses: what decides the outcome? - PubMed (original) (raw)
Review
Immunity and immunopathology to viruses: what decides the outcome?
Barry T Rouse et al. Nat Rev Immunol. 2010 Jul.
Abstract
Many viruses infect humans and most are controlled satisfactorily by the immune system with limited damage to host tissues. Some viruses, however, do cause overt damage to the host, either in isolated cases or as a reaction that commonly occurs after infection. The outcome is influenced by properties of the infecting virus, the circumstances of infection and several factors controlled by the host. In this Review, we focus on host factors that influence the outcome of viral infection, including genetic susceptibility, the age of the host when infected, the dose and route of infection, the induction of anti-inflammatory cells and proteins, as well as the presence of concurrent infections and past exposure to cross-reactive agents.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
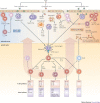
Figure 1. Immunity or immunopathology following viral infection.
Following entry into host cells, viruses (cytopathic or non-cytopathic) replicate at the site of infection. Cytopathic viruses kill infected cells, causing the release of cellular contents, including proteases and lysosomal enzymes, which digest the extracellular matrix and create an inflammatory milieu. Neutrophils that are rapidly recruited to the site of infection release inflammatory mediators. Innate cells recognize viral replication intermediates and secrete pro-inflammatory cytokines, which, in addition to helping to clear the virus, contribute to tissue damage. Viral antigens are taken up by antigen-presenting cells and carried to local draining lymph nodes. Depending on the cytokine milieu created in the draining lymph node, different types of T helper (TH) cell responses are induced. Primed CD8+ cytotoxic T lymphocytes (CTLs) migrate to the site of infection and kill virally infected cells, thereby contributing to tissue damage. After migrating to the site of infection, TH cells also contribute to the tissue damage. In conditions in which the control of aggressive TH cells and CTLs by regulatory T (TReg) cells is impaired and other inhibitory pathways fail to curtail them, tissue damage is the main consequence of viral infection. TH cells also provide help to B cells to secrete antibodies, which form immune complexes that are deposited in certain tissues such as the glomeruli of the kidneys and blood vessels to cause immune complex-mediated disease. DAMP, danger-associated molecular pattern; DC, dendritic cell; HBV, hepatitis B virus; HCV, hepatitis C virus; HSV, herpes simplex virus; IFN, interferon; IL, interleukin; MMP, matrix metalloproteinase; NK, natural killer; PAMP, pathogen-associated molecular pattern; pDC, plasmacytoid DC; RNS, reactive nitrogen species; ROS, reactive oxygen species; RSV, respiratory syncytial virus; TCR, T cell receptor; TFH, T follicular helper; TGFβ, transforming growth factor-β; TMEV, Theiler's murine encephalomyelitis virus; TNF, tumour necrosis factor.
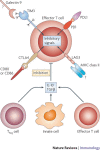
Figure 2. Inhibitory mechanisms to limit tissue damage caused by T cells.
Effector T cells upregulate inhibitory receptors such as programmed cell death 1 (PD1), T cell immunoglobulin domain and mucin domain protein 3 (TIM3), lymphocyte activation gene 3 (LAG3) and cytotoxic T lymphocyte antigen 4 (CTLA4) (and others such as adenosine receptors (not shown)) on their surface. Ligation of these receptors with PDL1, galectin 9, MHC class II molecules and CD80 or CD86, respectively, delivers inhibitory signals to the effector T cells and controls their inflammatory activity and subsequent tissue damage. In addition, activated regulatory T (TReg) cells, specialized innate cells or highly polarized effector T cells that can produce anti-inflammatory cytokines inhibit effector T cell responses. Inadequate control exerted by these pathways under some circumstances therefore results in uncontrolled T cell activation and proliferation causing excessive tissue damage. Question marks indicate interactions for which extensive in vivo studies have not been carried out. IL-10; interleukin-10; TGFβ, transforming growth factor-β.
Figure 3. Balance between pro-inflammatory and anti-inflammatory mechanisms may decide the outcome of viral infection.
a | Pro-inflammatory and anti-inflammatory mechanisms induced after viral infection. b | The balance between immunity and immunopathology following viral infection might depend on the levels of anti-inflammatory and pro-inflammatory mechanisms. A balanced combination of pro-inflammatory and anti-inflammatory mechanisms would facilitate viral clearance and immunity to reinfection, with minimal damage to host tissues. An excess of pro-inflammatory mechanisms would ensure viral clearance but causes tissue damage. If anti-inflammatory mechanisms outweigh pro-inflammatory mechanisms, the pathogen could persist in the host as a subclinical infection, an opportunist or a tissue-damaging agent. CCL2, CC-chemokine ligand 2; CTLA4, cytotoxic T lymphocyte antigen 4; CXCL2, CXC-chemokine ligand 2; DUBA, deubiquitinase enzyme A; IFN, interferon; IL, interleukin; IRAKM, IL-1R-associated kinase M; LAG3, lymphocyte activation gene 3; NLRX1, NOD-like receptor X1; PD1, programmed cell death 1; RNF125, ring finger containing domain 125; RLR, RIG-I-like receptor; SOCS, suppressor of cytokine signalling; TGFβ, transforming growth factor-β; TIM3, T cell immunoglobulin domain and mucin domain protein 3; TLR, Toll-like receptor; TNF, tumour necrosis factor; TReg, regulatory T.
Similar articles
- Contribution of cytokines to pathology and protection in virus infection.
Wack A, Openshaw P, O'Garra A. Wack A, et al. Curr Opin Virol. 2011 Sep;1(3):184-95. doi: 10.1016/j.coviro.2011.05.015. Epub 2011 Jun 25. Curr Opin Virol. 2011. PMID: 22440716 Review. - Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung.
Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK. Chen HD, et al. Am J Pathol. 2003 Oct;163(4):1341-55. doi: 10.1016/S0002-9440(10)63493-1. Am J Pathol. 2003. PMID: 14507643 Free PMC article. - Immune Ecosystem of Virus-Infected Host Tissues.
Maarouf M, Rai KR, Goraya MU, Chen JL. Maarouf M, et al. Int J Mol Sci. 2018 May 6;19(5):1379. doi: 10.3390/ijms19051379. Int J Mol Sci. 2018. PMID: 29734779 Free PMC article. Review. - Host Defenses to Viruses: Lessons from Inborn Errors of Immunity.
Leonardi L, Rivalta B, Leone F, Cancrini C, Caffarelli C, Marseglia GL, Cardinale F. Leonardi L, et al. Medicina (Kaunas). 2022 Feb 7;58(2):248. doi: 10.3390/medicina58020248. Medicina (Kaunas). 2022. PMID: 35208572 Free PMC article. Review. - [Interaction of viruses and Toll-like receptors].
Gankovskaia OA, Zverev VV. Gankovskaia OA, et al. Zh Mikrobiol Epidemiol Immunobiol. 2010 Mar-Apr;(2):101-5. Zh Mikrobiol Epidemiol Immunobiol. 2010. PMID: 20468100 Review. Russian.
Cited by
- Predicting viral proteins that evade the innate immune system: a machine learning-based immunoinformatics tool.
Beltrán JF, Belén LH, Yáñez AJ, Jimenez L. Beltrán JF, et al. BMC Bioinformatics. 2024 Nov 9;25(1):351. doi: 10.1186/s12859-024-05972-7. BMC Bioinformatics. 2024. PMID: 39522017 Free PMC article. - Charting Peptide Shared Sequences Between 'Diabetes-Viruses' and Human Pancreatic Proteins, Their Structural and Autoimmune Implications.
James SA, Joshua IA. James SA, et al. Bioinform Biol Insights. 2024 Nov 5;18:11779322241289936. doi: 10.1177/11779322241289936. eCollection 2024. Bioinform Biol Insights. 2024. PMID: 39502449 Free PMC article. - Regulatory role of microRNAs in virus-mediated inflammation.
Bannazadeh Baghi H, Bayat M, Mehrasa P, Alavi SMA, Lotfalizadeh MH, Memar MY, Taghavi SP, Zarepour F, Hamblin MR, Sadri Nahand J, Hashemian SMR, Mirzaei H. Bannazadeh Baghi H, et al. J Inflamm (Lond). 2024 Nov 4;21(1):43. doi: 10.1186/s12950-024-00417-7. J Inflamm (Lond). 2024. PMID: 39497125 Free PMC article. Review. - Metabolic reprogramming tips vaccinia virus infection outcomes by stabilizing interferon-γ induced IRF1.
Chang T, Alvarez J, Chappidi S, Crockett S, Sorouri M, Orchard RC, Hancks DC. Chang T, et al. PLoS Pathog. 2024 Oct 30;20(10):e1012673. doi: 10.1371/journal.ppat.1012673. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39475961 Free PMC article. - MicroRNAs are enriched at COVID-19 genomic risk regions, and their blood levels correlate with the COVID-19 prognosis of cancer patients infected by SARS-CoV-2.
Anfossi S, Darbaniyan F, Quinlan J, Calin S, Shimizu M, Chen M, Rausseo P, Winters M, Bogatenkova E, Do KA, Martinez I, Li Z, Antal L, Olariu TR, Wistuba I, Calin GA. Anfossi S, et al. Mol Cancer. 2024 Oct 21;23(1):235. doi: 10.1186/s12943-024-02094-9. Mol Cancer. 2024. PMID: 39434078 Free PMC article.
References
- de Martel C, Franceschi S. Infections and cancer: established associations and new hypotheses. Crit. Rev. Oncol. Hematol. 2009;70:183–194. - PubMed
- Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. - PubMed
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature Immunol. 2004;5:987–995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI063365/AI/NIAID NIH HHS/United States
- R01 EY005093/EY/NEI NIH HHS/United States
- R01 AI 106336501/AI/NIAID NIH HHS/United States
- R01 EY 05093/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous