Interplay between Cdh1 and JNK activity during the cell cycle - PubMed (original) (raw)
Interplay between Cdh1 and JNK activity during the cell cycle
Gustavo J Gutierrez et al. Nat Cell Biol. 2010 Jul.
Abstract
The ubiquitin ligase APC/C(Cdh1) coordinates degradation of key cell cycle regulators. We report here that a nuclear-localized portion of the stress-activated kinase JNK is degraded by the APC/C(Cdh1) during exit from mitosis and the G1 phase of the cell cycle. Expression of a non-degradable JNK induces prometaphase-like arrest and aberrant mitotic spindle dynamics. Moreover, JNK phosphorylates Cdh1 directly, during G2 and early mitosis, changing its subcellular localization and attenuating its ability to activate the APC/C during G2/M. This regulatory mechanism between JNK and Cdh1 reveals an important function for JNK during the cell cycle.
Figures
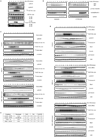
Figure 1. JNK is degraded in vivo and in vitro in a cell cycle- and KEN box-dependent manner
(A) Multi-alignment of a selected protein sequence from the ten members of the human JNK family. Highlighted in italics and underlined is the activation loop (amino acids 183–185, in JNK2α2); in bold and underlined is the KEN box (amino acids 203–205, in JNK2α2) and underlined a putative Destruction-box (D-box) (amino acids 295–298, in JNK2α2). (B) Extracts prepared from HeLa cells were synchronized by a double-thymidine block (DTB) and analyzed over a period of 18 h by immunoblotting using the indicated antibodies. JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+). FACS analysis confirming the synchronization is shown in Figure S1A. (C) Synchronized HeLa cells overexpressing either HA-tagged JNK2 (wild-type or mutants) or HA-tagged JIP1 (JNK Interacting Protein 1) or control-transfected cells were analyzed by immunoblotting with antibodies against HA-tag, cyclin B1, or tubulin. Quantification of JNK2 (wild-type or mutants) levels for each synchronization is shown in the graph. (D) JNK2 in vitro degradation assays in concentrated extracts prepared from HeLa cells released after being synchronized by either DTB (left panels) or nocodazole arrest (right panels). FACS data is included in Figure S1G. (E) JNK2 in vitro degradation assays in Xenopus laevis egg extracts. Uncropped images for key results of this figure are shown in Figure S7.
Figure 2. JNK levels are directly regulated by APC/CCdh1-mediated protein degradation during the cell cycle
(A) Top panels: in vitro binding assay using recombinant 6×His-tagged JNKs and Cdh1/fzr translated in reticulocyte lysates and radiolabeled with 35S-methionine. Bottom panels: in vitro binding assay using recombinant 6×His-tagged Cdh1 and JNK2 translated in reticulocyte lysates and radiolabeled. Autoradiograms and Coomassie-stained gels are shown. (B) In vivo binding between endogenous JNKs and endogenous Cdh1 immunoprecipitated from synchronized HeLa cells released from a double-thymidine block (DTB). JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+). (C) In vitro ubiquitination assay using JNK2 (wild-type or mutants) and immunoprecipitated APC/C complex from exponentially growing HeLa cells supplemented with Cdh1. (D) Overexpression of myc-tagged Cdh1 induces JNK degradation in HeLa cells. Time-course refers to hours after transfection of cells with Cdh1. Graph shows a quantification of the JNK signal. (E) Cell-cycle-synchronized Cdh1 RNAi’d HeLa cells were analyzed by immunoblotting for expression levels of Cdh1 and JNK. Uncropped images for key results of this figure are shown in Figure S7.
Figure 3. JNK activation during the cell cycle regulates its subcellular localization and degradation
(A) In vitro degradation assays, performed in Xenopus laevis interphase egg extracts supplemented with recombinant Cdh1, of FLAG-tagged JNK2 proteins (either wild-type or mutants) isolated from HeLa cells transfected for 48 h. Cells were either untreated (Control), UV-irradiated (45 J/m2) and harvested after 45 min (UV) or synchronized by DTB and harvested before mitosis (6 h after release) (G2). Extracts from these cells were separated into nuclear or cytosolic fractions when indicated. The different extracts were subjected to anti-FLAG immunoprecipitation. The bound material was eluted with the help of a saturating concentration of FLAG peptide, quantified and volume-adjusted before being used in the degradation assays. Signal observed was detected by FLAG immunoblotting and quantified with the help of the Odyssey software (LiCOR Biosciences). Numbers on the right depict the percentage of JNK2 protein degradation for each assay at the 120 min time point. Levels of JunB (whose reduction is a marker of G2-phase) were assessed in the double-thymidine blocked (DTB) versus 6 hours-released G2 extracts. FACS analyses are also included. (B) JNK activity (as detected, by either p-JNK blot or in vitro kinase assay using immunoprecipitated JNK) during the cell cycle in synchronized HeLa cells after release from a double-thymidine block (DTB). (C) HeLa cells synchronized by DTB at early S-phase, were harvested at the indicated times after release and biochemically separated into nuclear and cytosolic fractions. Each fraction was then analyzed with the indicated antibodies by immunoblotting. B23/nucleophosmin and GAPDH serve as nuclear and cytosolic markers, respectively. In this figure, JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+).Uncropped images for key results of this figure are shown in Figure S7.
Figure 4. Unrestricted activation of JNK during cell cycle progression regulates Wee1’s levels, Cdk1 activity, and entry into mitosis
(A) Biochemical cell cycle analyses of HFF-1 cells transfected with empty plasmid (Control), JNK2 wild-type (wt) or the JNK2ΔKEN mutant after DTB and release. Levels of overexpressed FLAG-tagged JNKs were analyzed by immunoblot together with levels of cyclin B1, phosphorylated-histone H3 at Serine 10 (p-histone H3) and tubulin (as loading control). Immunokinase assays of JNK (using GST-Nt-c-Jun as substrate) and Cdk1 (using a 6×His-tagged kinase-dead Wee1 as substrate) were performed. FACS data is included in Figure S4A. (B) Similar experiments as described in (A) but using HeLa cells. Levels of overexpressed FLAG-tagged JNKs were analyzed by immunoblot together with levels of cyclin B1, Wee1 and actin (as loading control). Immunokinase assays of JNK (using GST-Nt-c-Jun as substrate) and Cdk1 (using histone H1 as substrate) were performed. (A and B) Degradation pattern of JNK2-wt along the cell cycle is not obvious due to the efficient expression of JNK2 when using the pEF-FLAG plasmid (see Figures S1D–E for details). (C) Flow cytometry cell cycle analyses performed in HFF-1 cells overexpressing the indicated constructs under the stated treatments (noc: nocodazole treatment −18 hours). The percentage of cells in G1, S, and G2/M (a mixed population of cells in G2 and mitosis) phases of the cell cycle are included for one representative experiment. Experiments were repeated at least three times. Uncropped images for key results of this figure are shown in Figure S7.
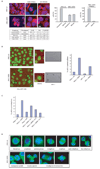
Figure 5. Hyperactivation of JNK during unperturbed cell cycle induces aberrant microtubular and chromosomal structures and a prometaphase-like arrest in cells
(A) Immunofluorescence microscopy performed in HFF-1 cells. Upper left panels depicts normal spindles in cells expressing JNK2 wild-type. Bottom left panels shows cells arrested in early mitosis and abnormal microtubular structures seen upon expression of JNK2ΔKEN. Tubulin is visualized in red and DNA in blue. Graphs on the right panels correspond to the G2/M (mitosis) arrest quantification observed by microscopy in HFF-1 cells expressing JNK2ΔKEN (n = 900 cells counted) versus JNK2 wild-type (n = 1200 cells counted) and the penetrance of the aberrant microtubular structures found in the cells arrested in the mitosis-like state. (B) Flow cytometry cell cycle analyses performed in HFF-1 cells overexpressing the indicated constructs under the stated treatments (noc: nocodazole −18 hours), for a representative experiment used to perform the microscopy depicted in (A). The percentage of cells in G1, S, and G2/M (a mixed population of G2 and mitosis) phases of the cell cycle are included. Experiments were repeated at least three times. (C) Top panels, captions taken from live imaging movies using either HeLa cells stably transfected with GFP-H2B or HFF-1 cells after overexpression of the indicated JNKs (for 24 h). Bottom panel, quantification of the prometaphase-like arrest induced by JNK2ΔKEN expression (for 24 h) in HeLa and HFF-1 cells. (D) Quantification of the percentage of HFF-1 cells in prometaphase-like arrest (as detected by immunofluorescence analysis after 24 h) under the conditions indicated. APF refers to a kinase-dead version of JNK2 (Thr183Ala, Tyr185Phe mutant). [i]-n corresponds to three different concentrations (n=1–3) of JNK inhibitor VII (100 nM, 0.5 µM, and 1 µM) used for 16 h. (E) Immunofluorescence analysis of mitotic spindles in HeLa transfected (for 48 h) with JNK2 wild-type (wt) or KEN-deleted mutant (JNK2-ΔKEN). Tubulin is shown in green and DNA (chromosomal) staining with DAPI in blue.
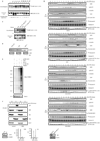
Figure 6. JNK-mediated phosphorylation of Cdh1 regulates its function
(A) In vitro kinase assays using active JNK and MBP-Cdh1 wild-type (wt) or single phosphorylation sites mutants. (B) In vitro kinase assays using active JNK and Cdk1 either alone or sequentially (first reaction –priming– was performed using cold ATP) and recombinant MBP-Cdh1 as substrate. (C) Unphosphorylated or in vitro phosphorylated 6×His-Cdh1 by JNK, were used to pull-down Cdc27 from extracts produced from exponentially growing cell lines. (D) In vitro ubiquitination assay using APC/C complex immunoprecipitated from HeLa cells and either unphosphorylated or JNK-phosphorylated Cdh1 and 6×His-TOME-1 as substrate. Ubiquitination reactions were performed in the presence of 32P-labeled ubiquitin (previously phosphorylated by PKA in vitro) and were analyzed by SDS-PAGE and PhosphorImaging. Membrane was probed with TOME-1 antibodies to detect levels of unmodified substrate. (E) Nuclear (N) and Cytosolic (C) fractions produced from HeLa cells synchronized by a DTB and released (0, early S-phase arrest; and 6 h, G2 phase) into control or JNK VII inhibitor-containing media (JNKi; 10 µM added at 4 hours release time-point). Extracts prepared from these fractions were analyzed by Cdc27 immunoblotting and subjected to Cdh1 immunoprecipitation followed by Cdh1 and phospho-ThrPro (pTP) immunoblotting. B23/nucleophosmin and PI3K serve as nuclear and cytoplasmic markers, respectively. Levels of JunB (whose reduction is a marker of G2-phase) were assessed in the 0 h (double-thymidine blocked) versus 6 h-released (G2) extracts, only for the control conditions. FACS analyses are also included. Uncropped images for key results of this figure are shown in Figure S7.
Figure 7. JNK phosphorylates Cdh1 in cells independently of CDKs activation
Phosphorylation status of endogenous Cdh1 after immunoprecipitation at residues Threonine 32 (T32) and Serine 36 (S36) in cell cycle-synchronized HeLa cells after a double-thymidine block (DTB). JNKi (JNK VII inhibitor) was used at 10 µM at the 4 hours release time-point. Roscovitine was utilized at 100 µM at the 6 hours release time-point. Down-regulation of JNK1 and JNK2 achieved by means of shRNA, is shown in the inset panels (bottom right). Western-blot corresponds to the 0 h time-point; JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+). CDKs assays were performed in vitro –using total extracts– by assessing 32P-γ-ATP incorporation in histone H1 as substrate. JNK activity was assessed in vitro, using total extracts incubated with cold ATP and recombinant GST-N-terminus-tagged c-Jun, and revealed by immunoblotting using phospho-Ser63-c-Jun antibodies following a GSH-pull down. Uncropped images for key results of this figure are shown in Figure S7.
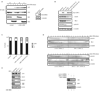
Figure 8. JNK-mediated phosphorylation of Cdh1 affects cell cycle progression
(A) Nuclear (N) and Cytosolic (C) fractions produced from HeLa cells, expressing either control or pEF-FLAG-JNK2α2-ΔKEN plasmids, were synchronized by a DTB and release (0; early S-phase arrest and 6 h; G2 phase) and analyzed by Cdh1 immunoprecipitation followed by Cdh1 and phospho-ThrPro (pTP) immunoblotting. B23/nucleophosmin and PI3K serve as nuclear and cytoplasmic markers, respectively. (B) Immunoblot analysis of Cdc20, Plk-1, and cyclin B1 protein levels in cells expressing for 36 h either Cdh1 wild-type (wt) or non-phosphorylatable triple mutants: (i) Threonine 32, Serines 36 and 151 to Alanine (a protein that cannot be phosphorylated by JNK) or (ii) Serines 40 and 70 and Threonine 121 to Alanine (a protein that is significantly less phosphorylated in vitro by Cdk2, see Figure S21). (C) Flow cytometry cell cycle analysis performed in HeLa cells overexpressing the indicated constructs. The percentage of cells in G1, S, and G2/M (a mixed population of G2 and mitosis) phases of the cell cycle are included. (D) HeLa cells infected, with either control or JNK1/2 shRNAs for 24 h, were cell cycle-synchronized by a double-thymidine block (DTB). Cells were also transfected after the first thymidine treatment with either control or Cdh1 shRNAs. Levels of JNK, Cdh1, and tubulin were assessed at time zero before release (bottom panels) and changes in cyclin B1 and Plk-1 levels and tubulin (as loading control) were analyzed during a cell cycle kinetic of 18 h. (E) Extracts from MEFs isolated from either wild-type or JNK1/2 DKO animals were analyzed by immunoblotting using antibodies directed against major cell cycle regulators (as shown), under downregulation of Cdh1 for 36 h by means of shRNA where indicated. In this figure, JNK2 displays as a 54kDa band (*) while JNK1 displays as a 46kDa band (+).Uncropped images for key results of this figure are shown in Figure S7.
Similar articles
- APC/C(Cdh1)-dependent degradation of Cdc20 requires a phosphorylation on CRY-box by Polo-like kinase-1 during somatic cell cycle.
Hyun SY, Sarantuya B, Lee HJ, Jang YJ. Hyun SY, et al. Biochem Biophys Res Commun. 2013 Jun 21;436(1):12-8. doi: 10.1016/j.bbrc.2013.04.073. Epub 2013 May 3. Biochem Biophys Res Commun. 2013. PMID: 23643811 - KEN-box-dependent degradation of the Bub1 spindle checkpoint kinase by the anaphase-promoting complex/cyclosome.
Qi W, Yu H. Qi W, et al. J Biol Chem. 2007 Feb 9;282(6):3672-9. doi: 10.1074/jbc.M609376200. Epub 2006 Dec 11. J Biol Chem. 2007. PMID: 17158872 - CKAP2 is a spindle-associated protein degraded by APC/C-Cdh1 during mitotic exit.
Seki A, Fang G. Seki A, et al. J Biol Chem. 2007 May 18;282(20):15103-13. doi: 10.1074/jbc.M701688200. Epub 2007 Mar 21. J Biol Chem. 2007. PMID: 17376772 - Control of mitotic transitions by the anaphase-promoting complex.
Fang G, Yu H, Kirschner MW. Fang G, et al. Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1583-90. doi: 10.1098/rstb.1999.0502. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582244 Free PMC article. Review. - The anaphase-promoting complex/cyclosome (APC/C): cell-cycle-dependent and -independent functions.
Manchado E, Eguren M, Malumbres M. Manchado E, et al. Biochem Soc Trans. 2010 Feb;38(Pt 1):65-71. doi: 10.1042/BST0380065. Biochem Soc Trans. 2010. PMID: 20074037 Review.
Cited by
- Selenite inhibits glutamine metabolism and induces apoptosis by regulating GLS1 protein degradation via APC/C-CDH1 pathway in colorectal cancer cells.
Zhao J, Zhou R, Hui K, Yang Y, Zhang Q, Ci Y, Shi L, Xu C, Huang F, Hu Y. Zhao J, et al. Oncotarget. 2017 Mar 21;8(12):18832-18847. doi: 10.18632/oncotarget.13600. Oncotarget. 2017. PMID: 27902968 Free PMC article. - Functional characterization of Anaphase Promoting Complex/Cyclosome (APC/C) E3 ubiquitin ligases in tumorigenesis.
Zhang J, Wan L, Dai X, Sun Y, Wei W. Zhang J, et al. Biochim Biophys Acta. 2014 Apr;1845(2):277-93. doi: 10.1016/j.bbcan.2014.02.001. Epub 2014 Feb 22. Biochim Biophys Acta. 2014. PMID: 24569229 Free PMC article. Review. - WD40-repeat protein 62 is a JNK-phosphorylated spindle pole protein required for spindle maintenance and timely mitotic progression.
Bogoyevitch MA, Yeap YY, Qu Z, Ngoei KR, Yip YY, Zhao TT, Heng JI, Ng DC. Bogoyevitch MA, et al. J Cell Sci. 2012 Nov 1;125(Pt 21):5096-109. doi: 10.1242/jcs.107326. Epub 2012 Aug 16. J Cell Sci. 2012. PMID: 22899712 Free PMC article. - Galectin-7 Regulates Keratinocyte Proliferation and Differentiation through JNK-miR-203-p63 Signaling.
Chen HL, Chiang PC, Lo CH, Lo YH, Hsu DK, Chen HY, Liu FT. Chen HL, et al. J Invest Dermatol. 2016 Jan;136(1):182-191. doi: 10.1038/JID.2015.366. J Invest Dermatol. 2016. PMID: 26763438 Free PMC article. - Role of JNK during buccopharyngeal membrane perforation, the last step of embryonic mouth formation.
Houssin NS, Bharathan NK, Turner SD, Dickinson AJ. Houssin NS, et al. Dev Dyn. 2017 Feb;246(2):100-115. doi: 10.1002/dvdy.24470. Epub 2016 Dec 29. Dev Dyn. 2017. PMID: 28032936 Free PMC article.
References
- Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. - PubMed
- Pines J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 2006;16:55–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous