Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice - PubMed (original) (raw)
Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice
Klaus Fabel et al. Front Neurosci. 2009.
Abstract
Voluntary physical exercise (wheel running, RUN) and environmental enrichment both stimulate adult hippocampal neurogenesis but do so by different mechanisms. RUN induces precursor cell proliferation, whereas ENR exerts a survival-promoting effect on newborn cells. In addition, continued RUN prevented the physiologically occurring age-related decline in precursor cell in the dentate gyrus but did not lead to a corresponding increase in net neurogenesis. We hypothesized that in the absence of appropriate cognitive stimuli the potential for neurogenesis could not be realized but that an increased potential by proliferating precursor cells due to RUN could actually lead to more adult neurogenesis if an appropriate survival-promoting stimulus follows the exercise. We thus asked whether a sequential combination of RUN and ENR (RUNENR) would show additive effects that are distinct from the application of either paradigm alone. We found that the effects of 10 days of RUN followed by 35 days of ENR were additive in that the combined stimulation yielded an approximately 30% greater increase in new neurons than either stimulus alone, which also increased neurogenesis. Surprisingly, this result indicates that although overall the amount of proliferating cells in the dentate gyrus is poorly predictive of net adult neurogenesis, an increased neurogenic potential nevertheless provides the basis for a greater efficiency of the same survival-promoting stimulus. We thus propose that physical activity can "prime" the neurogenic region of the dentate gyrus for increased neurogenesis in the case the animal is exposed to an additional cognitive stimulus, here represented by the enrichment paradigm.
Keywords: hippocampus; learning; reserve; stem cell.
Figures
Figure 1
Experimental design. During phase 1 of the experiment mice were either given access to a RUN ad libitum (RUNSTD and RUNENR group) or were housed under standard laboratory conditions (STDSTD and STDENR group). Dividing hippocampal precursor cells were labeled with BrdU during the last 3 days of phase 1. Subsequently, in phase 2 (duration: 35 days), animals were either exposed to an enriched environment (STDENR and RUNENR groups) or housed under standard laboratory conditions (STDSTD and RUNSTD group). Animals were killed after phase 2 was completed and brain tissue was prepared for immunohistochemistry. For further details see Sections “Introduction and Materials and Methods”.
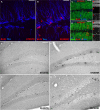
Figure 2
Activity-induced changes in the number of BrdU-labeled cells and new neurons in the dentate gyrus. (A,B) Confocal projections of the dentate gyrus (z-stack of 10 optical sections with 1.7-μm thickness). BrdU, Red; DCX, Blue. Scale bar, 25 μm in (A–D) Confocal image (optical section of 1-μm thickness) of STDSTD in (C) and RUNENR in (D) showing NeuN, Green; BrdU, Red, S100β, Blue; Inset: a, NeuN; b, BrdU; c, S100β. (D) Arrowheads in red indicating colocalization of BrdU and NeuN. Scale bar, 25 μm in (C,D). (E–H) Brightfield images of BrdU-positive cells in the dentate gyrus. Scale bar (in E for E–H), 100 μm.
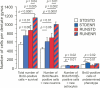
Figure 3
The number of BrdU-labeled cells in the dentate gyrus was determined along with the cellular phenotypes of BrdU-positive cells (NeuN indicating a neuronal, S100β an astroglial fate). We found RUNSTD to cause an increased number of BrdU-labeled cells as well as new neurons compared to STDSTD. RUNENR resulted in a further increase in the number of BrdU-positive cells and newborn neurons. Therefore, the effects of wheel running and environmental enrichment on adult hippocampal neurogenesis were additive. Fisher post hoc test after ANOVA.
Similar articles
- p27kip1 Is Required for Functionally Relevant Adult Hippocampal Neurogenesis in Mice.
Hörster H, Garthe A, Walker TL, Ichwan M, Steiner B, Khan MA, Lie DC, Nicola Z, Ramirez-Rodriguez G, Kempermann G. Hörster H, et al. Stem Cells. 2017 Mar;35(3):787-799. doi: 10.1002/stem.2536. Epub 2016 Nov 28. Stem Cells. 2017. PMID: 27790794 - Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease.
Mirochnic S, Wolf S, Staufenbiel M, Kempermann G. Mirochnic S, et al. Hippocampus. 2009 Oct;19(10):1008-18. doi: 10.1002/hipo.20560. Hippocampus. 2009. PMID: 19219917 - Environmental enrichment: dissociated effects between physical activity and changing environmental complexity on anxiety and neurogenesis in adult male Balb/C mice.
Ramírez-Rodríguez GB, Gutiérrez-Vera B, Ortiz-López L, Vega-Rivera NM, Juan DM, Granados-Juárez A, Aquino DVC, Castro-García M, Ramos MF. Ramírez-Rodríguez GB, et al. Physiol Behav. 2022 Oct 1;254:113878. doi: 10.1016/j.physbeh.2022.113878. Epub 2022 Jun 11. Physiol Behav. 2022. PMID: 35700814 - Control of the Cell Cycle in Adult Neurogenesis and its Relation with Physical Exercise.
Farioli-Vecchioli S, Tirone F. Farioli-Vecchioli S, et al. Brain Plast. 2015 Oct 9;1(1):41-54. doi: 10.3233/BPL-150013. Brain Plast. 2015. PMID: 29765834 Free PMC article. Review. - Different Mechanisms Must Be Considered to Explain the Increase in Hippocampal Neural Precursor Cell Proliferation by Physical Activity.
Overall RW, Walker TL, Fischer TJ, Brandt MD, Kempermann G. Overall RW, et al. Front Neurosci. 2016 Aug 3;10:362. doi: 10.3389/fnins.2016.00362. eCollection 2016. Front Neurosci. 2016. PMID: 27536215 Free PMC article. Review.
Cited by
- Cognitive and physical impact of combined exercise and cognitive intervention in older adults with mild cognitive impairment: A systematic review and meta-analysis.
Yi Q, Liu Z, Zhong F, Selvanayagam VS, Cheong JPG. Yi Q, et al. PLoS One. 2024 Oct 3;19(10):e0308466. doi: 10.1371/journal.pone.0308466. eCollection 2024. PLoS One. 2024. PMID: 39361605 Free PMC article. - Maintaining a Dynamic Brain: A Review of Empirical Findings Describing the Roles of Exercise, Learning, and Environmental Enrichment in Neuroplasticity from 2017-2023.
Milbocker KA, Smith IF, Klintsova AY. Milbocker KA, et al. Brain Plast. 2024 May 14;9(1-2):75-95. doi: 10.3233/BPL-230151. eCollection 2024. Brain Plast. 2024. PMID: 38993580 Free PMC article. Review. - Adolescent environmental enrichment induces social resilience and alters neural gene expression in a selectively bred rodent model with anxious phenotype.
O'Connor AM, Hagenauer MH, Thew Forrester LC, Maras PM, Arakawa K, Hebda-Bauer EK, Khalil H, Richardson ER, Rob FI, Sannah Y, Watson SJ Jr, Akil H. O'Connor AM, et al. Neurobiol Stress. 2024 May 30;31:100651. doi: 10.1016/j.ynstr.2024.100651. eCollection 2024 Jul. Neurobiol Stress. 2024. PMID: 38933284 Free PMC article. - The Influence of Separate and Combined Exercise and Foreign Language Acquisition on Learning and Cognition.
Qian Y, Schwartz A, Jung A, Zhang Y, Seitz U, Wilds G, Kim M, Kramer AF, Chukoskie L. Qian Y, et al. Brain Sci. 2024 Jun 3;14(6):572. doi: 10.3390/brainsci14060572. Brain Sci. 2024. PMID: 38928573 Free PMC article.
References
- Brandt M. D., Jessberger S., Steiner B., Kronenberg G., Reuter K., Bick-Sander A., von der Behrens W., Kempermann G. (2003). Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol. Cell. Neurosci. 24, 603–61310.1016/S1044-7431(03)00207-0 - DOI - PubMed
LinkOut - more resources
Full Text Sources