ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS - PubMed (original) (raw)
ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS
Shuo-Chien Ling et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Dominant mutations in two functionally related DNA/RNA-binding proteins, trans-activating response region (TAR) DNA-binding protein with a molecular mass of 43 KDa (TDP-43) and fused in sarcoma/translocation in liposarcoma (FUS/TLS), cause an inherited form of ALS that is accompanied by nuclear and cytoplasmic aggregates containing TDP-43 or FUS/TLS. Using isogenic cell lines expressing wild-type or ALS-linked TDP-43 mutants and fibroblasts from a human patient, pulse-chase radiolabeling of newly synthesized proteins is used to determine, surprisingly, that ALS-linked TDP-43 mutant polypeptides are more stable than wild-type TDP-43. Tandem-affinity purification and quantitative mass spectrometry are used to identify TDP-43 complexes not only with heterogeneous nuclear ribonucleoproteins family proteins, as expected, but also with components of Drosha microprocessor complexes, consistent with roles for TDP-43 in both mRNA processing and microRNA biogenesis. A fraction of TDP-43 is shown to be complexed with FUS/TLS, an interaction substantially enhanced by TDP-43 mutants. Taken together, abnormal stability of mutant TDP-43 and its enhanced binding to normal FUS/TLS imply a convergence of pathogenic pathways from mutant TDP-43 and FUS/TLS in ALS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
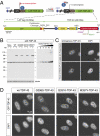
Characterization of isogenic cell lines expressing a single copy of wild-type and ALS-linked TDP-43 mutations. (A) Schematic representation of site-directed recombinase-based system to generate isogenic stable cell lines, in which CMV promoter was used to drive the expression of tetracycline (Tet)-inducible wild-type and mutant genes that were integrated at a common locus [Flp Recognition Target (FRT) site]. Normally, Tet repressor (TetR) binds to Tet operator (TetO), repressing transcription. On addition, binding of Tet to TetR induces a conformation change and releases TetR from TetO, allowing transcription to start. Lower shows the LAP tag of TDP-43. The LAP tag is composed of GFP followed by PreScission protease cleavage sequences and 6× histidine tag. TDP-43 is tagged at the N terminus with myc peptide (EQKLISSEEDL) and at the C terminus with HA peptide (YPYDVPDYA). Three different mutations, G298S, Q331K, and M337V, were used in this study. (B) Expression of transgene in isogenic stable cell lines. The transgenes are under TetR control. The transgenes express on incubating with tetracycline (−, without tetracycline; +, with tetracycline); 20 μg total cell extract was loaded for each lane. Recombinant TDP-43 of known amount is loaded for the quantification, and tubulin is used as loading control. Exposure shown was taken on the same blot for quantification. (C) Immunofluorescence images of TDP-43 in HeLa cells. Both rabbit polyclonal antibody (ProteinTech) and mouse monoclonal antibody (FL4) showed similar staining pattern for nuclear TDP bodies. (D) Fluorescent images of isogenic cell lines. Upper is GFP signal, and Lower is DAPI-stained to mark the nucleus. All LAP-tagged TDP-43 form nuclear speckles that are similar to immunofluorescence images of endogenous TDP-43 (C). (Scale bar, 10 μm.)
Fig. 2.
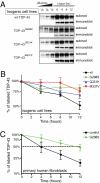
ALS-linked TDP-43 mutations exhibit longer half-lives. (A) Representative autoradiogram and immunoblots of the pulse-chase assay using isogenic cell lines expressing wild type (wt), G298S, Q331K, and M337V mutations in TDP-43. LAP-tagged wt–TDP-43 and its mutants were immunoprecipitated and run on SDS-PAGE for autoradiography and immunoblotting. Serial dilutions of 0-h point (start of chase) were used to monitor the linearity of the autoradiograph signal. The autoradiograph signals were normalized to the immunoblotting signals and plotted using 0 h as 100%. (B) Half-lives of LAP-tagged TDP-43 and its ALS-linked mutations (n = 5). Error bar represents SEM. (C) Half-lives of TDP-43 in primary human fibroblasts. Wild-type TDP-43 exhibits 4-h half-life, whereas TDP-43 in heterozygote harboring a copy of G298S mutant exhibits 11-h half-life (n = 5). Error bar represents SEM.
Fig. 3.
Quantitative proteomic analysis of TDP-43 using SILAC coupled with TAP. (A) Schematic representation of TAP purification and quantitative analysis using SILAC. (B) Samples from key steps (clarified lysate, immunoprecipitation for GFP (GFP-IP), PreScission elution, and final elution) in purification were probed with TDP-43 antibody, showing that tagged TDP-43 associates and copurifies with endogenous TDP-43. (C) Silver stain of the samples from the final elution of TAP step. Arrow indicates tagged TDP-43. (D) Representative mass spectrum for TDP-43 peptide. The corresponding light isotope-containing peptide is below detection limit, whereas the heavy Lys/Arg-containing peptide is detected. Asterisks indicate the natural occurrences of 13C/15N in the peptide, as revealed by high resolution mass spectrometry. (E) Confirmation of putative TDP-43–associated proteins by immunoblotting, including hnRNP Q, hnRNP A2/B1, hnRNP K, hnRNP H, and interleukin-enhancer binding factor 3/nuclear factor 90 KDa (ILF3/NF90). (F) Summary for putative TDP-43–associated proteins. TDP-associated proteins were identified with peptides containing only heavy Lys/Arg and by multiple different peptides from at least two independent runs. The associated proteins were grouped according to the known assigned functions for the proteins.
Fig. 4.
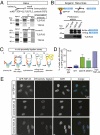
ALS-linked TDP-43 mutations associate with FUS/TLS. (A) Reciprocal immunoprecipitation shows that TDP-43 interacts with FUS/TLS using HeLa cells. A monoclonal antibody against splicing factor proline/glutamine-rich or polypyrimidine tract binding protein-associated splicing factor (SFPQ/PSF) was used for control. (B) PreScission elution fractions from GFP pull-down samples showed that FUS/TLS associates more prominently with Q331K and M337V mutations in TDP-43. (C) Schematic representation of in situ proximity ligation assay. (D) Quantification of in situ proximity ligation assay (PLA) results (n = 3; cell numbers > 100 per experiment). Error bar represents SEM. A combination of anti-myc antibody and rabbit IgG molecules was used as negative control, whereas a combination of anti-myc and anti–TDP-43 antibodies was used as positive control. The monitored signal used a combination of anti-myc and anti-TLS/FUS antibody. (E) Representative results for in situ proximity ligation assay. (Scale bar, 15 μm.)
Similar articles
- Amyotrophic lateral sclerosis-associated proteins TDP-43 and FUS/TLS function in a common biochemical complex to co-regulate HDAC6 mRNA.
Kim SH, Shanware NP, Bowler MJ, Tibbetts RS. Kim SH, et al. J Biol Chem. 2010 Oct 29;285(44):34097-105. doi: 10.1074/jbc.M110.154831. Epub 2010 Aug 18. J Biol Chem. 2010. PMID: 20720006 Free PMC article. - Understanding the role of TDP-43 and FUS/TLS in ALS and beyond.
Da Cruz S, Cleveland DW. Da Cruz S, et al. Curr Opin Neurobiol. 2011 Dec;21(6):904-19. doi: 10.1016/j.conb.2011.05.029. Epub 2011 Aug 2. Curr Opin Neurobiol. 2011. PMID: 21813273 Free PMC article. Review. - Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs.
Lagier-Tourenne C, Polymenidou M, Hutt KR, Vu AQ, Baughn M, Huelga SC, Clutario KM, Ling SC, Liang TY, Mazur C, Wancewicz E, Kim AS, Watt A, Freier S, Hicks GG, Donohue JP, Shiue L, Bennett CF, Ravits J, Cleveland DW, Yeo GW. Lagier-Tourenne C, et al. Nat Neurosci. 2012 Nov;15(11):1488-97. doi: 10.1038/nn.3230. Epub 2012 Sep 30. Nat Neurosci. 2012. PMID: 23023293 Free PMC article. - TDP-43 and FUS/TLS: sending a complex message about messenger RNA in amyotrophic lateral sclerosis?
Strong MJ, Volkening K. Strong MJ, et al. FEBS J. 2011 Oct;278(19):3569-77. doi: 10.1111/j.1742-4658.2011.08277.x. Epub 2011 Sep 6. FEBS J. 2011. PMID: 21810174 Review. - A Drosophila model of FUS-related neurodegeneration reveals genetic interaction between FUS and TDP-43.
Lanson NA Jr, Maltare A, King H, Smith R, Kim JH, Taylor JP, Lloyd TE, Pandey UB. Lanson NA Jr, et al. Hum Mol Genet. 2011 Jul 1;20(13):2510-23. doi: 10.1093/hmg/ddr150. Epub 2011 Apr 12. Hum Mol Genet. 2011. PMID: 21487023 Free PMC article.
Cited by
- The Impact of ALS-Associated Genes hnRNPA1, MATR3, VCP and UBQLN2 on the Severity of TDP-43 Aggregation.
Bajc Česnik A, Motaln H, Rogelj B. Bajc Česnik A, et al. Cells. 2020 Jul 28;9(8):1791. doi: 10.3390/cells9081791. Cells. 2020. PMID: 32731393 Free PMC article. - Linking RNA Dysfunction and Neurodegeneration in Amyotrophic Lateral Sclerosis.
Barmada SJ. Barmada SJ. Neurotherapeutics. 2015 Apr;12(2):340-51. doi: 10.1007/s13311-015-0340-3. Neurotherapeutics. 2015. PMID: 25689976 Free PMC article. Review. - Optineurin Deficiency and Insufficiency Lead to Higher Microglial TDP-43 Protein Levels.
Prtenjaca N, Rob M, Alam MS, Markovinovic A, Stuani C, Buratti E, Munitic I. Prtenjaca N, et al. Int J Mol Sci. 2022 Jun 19;23(12):6829. doi: 10.3390/ijms23126829. Int J Mol Sci. 2022. PMID: 35743272 Free PMC article. - The ALS-FTD-linked gene product, C9orf72, regulates neuronal morphogenesis via autophagy.
Ho WY, Tai YK, Chang JC, Liang J, Tyan SH, Chen S, Guan JL, Zhou H, Shen HM, Koo E, Ling SC. Ho WY, et al. Autophagy. 2019 May;15(5):827-842. doi: 10.1080/15548627.2019.1569441. Epub 2019 Jan 28. Autophagy. 2019. PMID: 30669939 Free PMC article. - Transcriptional targets of amyotrophic lateral sclerosis/frontotemporal dementia protein TDP-43 - meta-analysis and interactive graphical database.
Cao MC, Scotter EL. Cao MC, et al. Dis Model Mech. 2022 Sep 1;15(9):dmm049418. doi: 10.1242/dmm.049418. Epub 2022 Sep 13. Dis Model Mech. 2022. PMID: 35946434 Free PMC article.
References
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–S17. - PubMed
- Arai T, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. - PubMed
- Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
- Kabashi E, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AG 000216/AG/NIA NIH HHS/United States
- R37NS27036/NS/NINDS NIH HHS/United States
- P30 NS0471401-08/NS/NINDS NIH HHS/United States
- T32 AG000216/AG/NIA NIH HHS/United States
- RC1 NS069144/NS/NINDS NIH HHS/United States
- RC1NS069144/NS/NINDS NIH HHS/United States
- R01 GM080469/GM/NIGMS NIH HHS/United States
- 089701/WT_/Wellcome Trust/United Kingdom
- R37 NS027036/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous