Dynamic membrane remodeling at invadopodia differentiates invadopodia from podosomes - PubMed (original) (raw)
Dynamic membrane remodeling at invadopodia differentiates invadopodia from podosomes
Vira V Artym et al. Eur J Cell Biol. 2011 Feb-Mar.
Abstract
Invadopodia are specialized actin-rich protrusions of metastatic tumor and transformed cells with crucial functions in ECM degradation and invasion. Although early electron microscopy studies described invadopodia as long filament-like protrusions of the cell membrane adherent to the matrix, fluorescence microscopy studies have focused on invadopodia as actin-cortactin aggregates localized to areas of ECM degradation. The absence of a clear conceptual integration of these two descriptions of invadopodial structure has impeded understanding of the regulatory mechanisms that govern invadopodia. To determine the relationship between the membrane filaments identified by electron microscopy and the actin-cortactin aggregates of invadopodia, we applied rapid live-cell high-resolution TIRF microscopy to examine cell membrane dynamics at the cortactin core of the invadopodia of human carcinoma cells. We found that cortactin docking to the cell membrane adherent to 2D fibronectin matrix initiates invadopodium assembly associated with the formation of an invadopodial membrane process that extends from a ventral cell membrane lacuna toward the ECM. The tip of the invadopodial process flattens as it interacts with the 2D matrix, and it undergoes constant rapid ruffling and dynamic formation of filament-like protrusions as the invadopodium matures. To describe this newly discovered dynamic relationship between the actin-cortactin core and invadopodial membranes, we propose a model of the invadopodial complex. Using TIRF microscopy, we also established that - in striking contrast to the invadopodium - membrane at the podosome of a macrophage fails to form any process- or filament-like membrane protrusions. Thus, the undulation and ruffling of the invadopodial membrane together with the formation of dynamic filament-like extensions from the invadopodial cortactin core defines invadopodia as invasive superstructures that are distinct from the podosomes.
Copyright © 2010 Elsevier GmbH. All rights reserved.
Figures
Figure 1
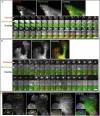
Cell membrane dynamics at invadopodia. Human breast carcinoma wt c-Src MDA-MB-231 cells expressing GFP-Cortactin and membrane marker IL2R-mCherry were cultured on a layer of FN. A. Initiation of invadopodium formation. An invadopodium in early stages of formation is shown selected with a region-of-interest (ROI) frame. The ROI montage shows every 6th frame, and the total duration of the time-lapse image acquisition used for the montage is 6.9 min. Scale bar indicates 3 μm. B. Mature invadopodium. This stage is characterized by a high degree of cell membrane morphological changes as exemplified by the invadopodial complex analyzed in the ROI. The montage of ROIs shows every 5th frame, and the total duration of the time-lapse image acquisition used for the montage is 5.3 min. Scale bar indicates 3 μm. C. Enlarged views of three frames from the ROI montage shown in B. White arrows point to filament-like membrane extensions that originate from the cortactin core; they also indicate the furthest extent of cortactin along the membrane filament-like protrusion. Yellow arrowheads point to membrane ruffles.
Figure 2
A. Dynamics of the cell membrane at the focal adhesion of HFF transiently expressing GFP-Vinculin and membrane marker IL2R-mCherry. HFF were cultured on a 2D FN matrix. A representative focal adhesion is highlighted by the ROI. The ROI montage shows every 10th frame of the time lapse of focal adhesion extension; the total duration of time-lapse image acquisition used for the montage is 12.7 min. Scale bar indicates 3 μm. B. Dynamics of the cell membrane at a podosome of IC-21 macrophage transiently expressing GFP-Cortactin and membrane marker IL2R-mCherry. Transfected macrophages were cultured on a 2D FN matrix. The ROI montage shows every 10th frame, and the total duration of the time-lapse image acquisition used for the montage is 5.2 min. Scale bar indicates 1 μm. C. TIRF microscopy of IC-21 macrophage expressing IL2R-mCherry and immuno-labeled with phalloidin-Alexa488 and anti-vinculin Cy-5-conjugated antibody. Enlargements of the ROI are shown as insets.
Figure 3
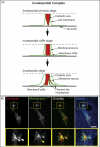
A, Model of the invadopodial complex. Dashed line indicates the upper boundary of the membrane-cytoplasmic zone visualized by TIRF microscopy. B, Invadopodial complex formation in wt c-Src MDA-MB-231 cells invading 3D matrix of 3 mg/ml rat tail collagen type I fluorescently labeled with Alexa647. MDA-MB-231 cells transiently expressing GFP-Cortactin and IL2R-mCherry were polymerized between two layers of rat tail collagen and allowed to invade 3D collagen matrix overnight. White arrows point to cortactin-rich invadopodial cores and yellow arrow points to filament-like invadopodia. Scale bar indicates 10 μm.
Similar articles
- Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function.
Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Artym VV, et al. Cancer Res. 2006 Mar 15;66(6):3034-43. doi: 10.1158/0008-5472.CAN-05-2177. Cancer Res. 2006. PMID: 16540652 - Phosphorylated cortactin recruits Vav2 guanine nucleotide exchange factor to activate Rac3 and promote invadopodial function in invasive breast cancer cells.
Rosenberg BJ, Gil-Henn H, Mader CC, Halo T, Yin T, Condeelis J, Machida K, Wu YI, Koleske AJ. Rosenberg BJ, et al. Mol Biol Cell. 2017 May 15;28(10):1347-1360. doi: 10.1091/mbc.E16-12-0885. Epub 2017 Mar 29. Mol Biol Cell. 2017. PMID: 28356423 Free PMC article. - The structure of invadopodia in a complex 3D environment.
Tolde O, Rösel D, Veselý P, Folk P, Brábek J. Tolde O, et al. Eur J Cell Biol. 2010 Sep;89(9):674-80. doi: 10.1016/j.ejcb.2010.04.003. Eur J Cell Biol. 2010. PMID: 20537759 - The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function.
Murphy DA, Courtneidge SA. Murphy DA, et al. Nat Rev Mol Cell Biol. 2011 Jun 23;12(7):413-26. doi: 10.1038/nrm3141. Nat Rev Mol Cell Biol. 2011. PMID: 21697900 Free PMC article. Review. - Digging a little deeper: the stages of invadopodium formation and maturation.
Beaty BT, Condeelis J. Beaty BT, et al. Eur J Cell Biol. 2014 Oct;93(10-12):438-44. doi: 10.1016/j.ejcb.2014.07.003. Epub 2014 Jul 21. Eur J Cell Biol. 2014. PMID: 25113547 Free PMC article. Review.
Cited by
- Matrix metalloproteinases: the gene expression signatures of head and neck cancer progression.
Iizuka S, Ishimaru N, Kudo Y. Iizuka S, et al. Cancers (Basel). 2014 Feb 13;6(1):396-415. doi: 10.3390/cancers6010396. Cancers (Basel). 2014. PMID: 24531055 Free PMC article. - Signaling inputs to invadopodia and podosomes.
Hoshino D, Branch KM, Weaver AM. Hoshino D, et al. J Cell Sci. 2013 Jul 15;126(Pt 14):2979-89. doi: 10.1242/jcs.079475. Epub 2013 Jul 10. J Cell Sci. 2013. PMID: 23843616 Free PMC article. Review. - Cortactin: a multifunctional regulator of cellular invasiveness.
Kirkbride KC, Sung BH, Sinha S, Weaver AM. Kirkbride KC, et al. Cell Adh Migr. 2011 Mar-Apr;5(2):187-98. doi: 10.4161/cam.5.2.14773. Epub 2011 Mar 1. Cell Adh Migr. 2011. PMID: 21258212 Free PMC article. Review. - Autocrine HBEGF expression promotes breast cancer intravasation, metastasis and macrophage-independent invasion in vivo.
Zhou ZN, Sharma VP, Beaty BT, Roh-Johnson M, Peterson EA, Van Rooijen N, Kenny PA, Wiley HS, Condeelis JS, Segall JE. Zhou ZN, et al. Oncogene. 2014 Jul 17;33(29):3784-93. doi: 10.1038/onc.2013.363. Epub 2013 Sep 9. Oncogene. 2014. PMID: 24013225 Free PMC article. - A new front in cell invasion: The invadopodial membrane.
Hastie EL, Sherwood DR. Hastie EL, et al. Eur J Cell Biol. 2016 Nov;95(11):441-448. doi: 10.1016/j.ejcb.2016.06.006. Epub 2016 Jun 24. Eur J Cell Biol. 2016. PMID: 27402208 Free PMC article. Review.
References
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971;67:359–367. - PubMed
- Akisaka T, Yoshida H, Suzuki R, Takama K. Adhesion structures and their cytoskeleton-membrane interactions at podosomes of osteoclasts in culture. Cell Tissue Res. 2008;331:625–641. - PubMed
- Akiyama SK. Curr Protoc Cell Biol. 2001. Purification of fibronectin; p. 15. Chapter 10, Unit 10. - PubMed
- Artym VV, Yamada KM, Mueller SC. ECM degradation assays for analyzing local cell invasion. Methods Mol Biol. 2009;522:211–219. - PubMed
- Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99CA129205/CA/NCI NIH HHS/United States
- K99 CA129205-02/CA/NCI NIH HHS/United States
- R01 CA112673-14/CA/NCI NIH HHS/United States
- K99 CA129205/CA/NCI NIH HHS/United States
- ZIA DE000719-04/ImNIH/Intramural NIH HHS/United States
- R01 CA112673/CA/NCI NIH HHS/United States
- Z01 DE000719/ImNIH/Intramural NIH HHS/United States
- K99 CA129205-02S1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous