Quantification of O-glycosylation stoichiometry and dynamics using resolvable mass tags - PubMed (original) (raw)
Quantification of O-glycosylation stoichiometry and dynamics using resolvable mass tags
Jessica E Rexach et al. Nat Chem Biol. 2010 Sep.
Abstract
Mechanistic studies of O-GlcNAc glycosylation have been limited by an inability to monitor the glycosylation stoichiometries of proteins obtained from cells. Here we describe a powerful method to visualize the O-GlcNAc-modified protein subpopulation using resolvable polyethylene glycol mass tags. This approach enables rapid quantification of in vivo glycosylation levels on endogenous proteins without the need for protein purification, advanced instrumentation or expensive radiolabels. In addition, it establishes the glycosylation state (for example, mono-, di-, tri-) of proteins, providing information regarding overall O-GlcNAc site occupancy that cannot be obtained using mass spectrometry. Finally, we apply this strategy to rapidly assess the complex interplay between glycosylation and phosphorylation and discover an unexpected reverse 'yin-yang' relationship on the transcriptional repressor MeCP2 that was undetectable by traditional methods. We anticipate that this mass-tagging strategy will advance our understanding of O-GlcNAc glycosylation, as well as other post-translational modifications and poorly understood glycosylation motifs.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
Figures
Figure 1
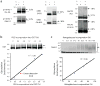
Mass-tagging strategy for quantifying _O_-GlcNAc glycosylation levels on specific proteins. (a) Schematic of the approach. _O_-GlcNAc-modified proteins are chemoenzymatically labeled using the UDP-ketogalactose analogue 1 and an engineered GalT enzyme, followed by reaction with an aminooxy-functionalized PEG mass tag (2 or 3). This approach enables facile visualization of _O_-GlcNAc-glycosylated species upon SDS-PAGE and immunoblotting with antibodies against proteins of interest. (b) Validation of the approach using CREBmono and CREBco. CREBmono was 28.2% glycosylated and existed primarily in the mono-glycosylated state, whereas CREBco was 88.8% glycosylated and present in multiply glycosylated forms. (c) _In vivo O_-GlcNAc stoichiometries vary significantly even among proteins with similar functions. Cell lysates (100 μg) from 293T cells (source of Sp1), adult rat brain (source of MeCP2, Nup62, and synapsin Ia (upper band) and IIa (lower band)), embryonic neuronal cultures (source of CREB, and OGA) or purified p75-OGT (0.43 μg) from Sf9 cells were subjected to chemoenzymatic labeling, SDS-PAGE, and immunoblotting with antibodies against the indicated proteins. See Methods for details. In all cases, 1 was excluded as a control for selectivity. The indicated glycosylation stoichiometries were determined by measuring the relative intensities of the glycosylated and nonglycosylated bands. (….) denotes the nonglycosylated protein fraction, (→) denotes _O_-GlcNAc glycosylated protein fraction shifted with 2 or 3. Full-length blots are presented in Supplementary Figure 9.
Figure 2
Validation of the approach. (a) Distinct antibodies detect the same glycosylation stoichiometry on OGT (AL-28 and DM-17), CREB (Chemicon 5432 and Upstate 06-863), and MeCP2 (Hu and Upstate 07-013). p110-OGT was expressed in Sf9 cells, endogenous CREB was from rat liver, and MeCP2 was co-expressed with OGT in Sf9 cells. (b) As little as 0.8% of glycosylated OGT is readily detected. Sf9 cell lysate containing over-expressed p110-OGT was chemoezymatically labeled with PEG derivative 3 and diluted with unlabeled lysate to generate standards with varying percentages of label incorporation. The lysate was resolved by SDS-PAGE and immunoblotted with the anti-OGT antibody DM-17. The limit of detection was defined as the lowest stoichiometry value within 10% of the linear fit. See Supplementary Methods for details. (c) Detection of PEG incorporation into ketogalactose-labeled Nup62 is linear across a wide range of stoichiometries (0–100%). 293T cell lysate was labeled with UDP-ketogalactose 1 and then diluted with varying amounts of unlabeled lysate to simulate different levels of glycosylation. Each mixture was reacted with 2, resolved by SDS-PAGE, and immunoblotted for Nup62. Full-length blots are presented in Supplementary Figure 9.
Figure 3
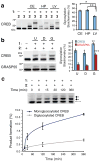
Monitoring _O_-GlcNAc glycosylation levels on proteins across various tissues and after cellular stimulation. (a) CREB is _O_-GlcNAc glycosylated at higher levels in the rat cerebellum (CE) and hippocampus (HP) than in the liver (LV). Cell lysates from the indicated tissues were chemoenzymatically labeled, resolved by SDS-PAGE, and immunoblotted for CREB. A representative immunoblot from one animal is shown. Plotted are the glycosylation stoichiometries of CREB averaged across multiple animals (n = 4–6). * P = 0.0003, ** P = 0.03. (b) _O_-GlcNAc glycosylation levels of CREB and GRASP55 decreased in rat embryonic neuronal cultures upon DON treatment (D) and increased upon GlcN treatment (G) relative to untreated neurons (U). Cell lysates were chemoenzymatically labeled, resolved by SDS-PAGE, and immunoblotted for the indicated proteins. A representative immunoblot is shown. Plotted are the glycosylation stoichiometries averaged across n = 3 experiments. * P = 0.002, ** P = 0.006, *** P = 0.001. (c) The rate constant for monoglycosylation of CREB is approximately threefold higher than that for diglycosylation of CREB. Neuro2a cells were treated with 10 mM GlcN for the indicated times. Crude nuclear protein lysates were chemoezymatically labeled, resolved by SDS-PAGE, and immunoblotted for CREB. Formation of monoglycosylated or diglycosylated CREB was plotted as a function of time for n = 3 experiments. See Supplementary Methods for details. Data represent mean ± s.e.m. Statistical analyses were performed using the Student’s _t_-test (two-tailed, paired). Full-length blots are presented in Supplementary Figure 9.
Figure 4
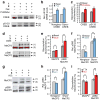
Dissecting the interplay between _O_-GlcNAc and phosphorylation. Detection of four subpopulations of CREB or MeCP2: glycosylated (1), nonglycosylated (2), glycosylated and phosphorylated (3), and nonglycosylated and phosphorylated (4). (a) CREB can be simultaneously glycosylated and phosphorylated. Mass-tagged lysates from 293T cells treated with Fsk, PUGNAc or vehicle were immunoblotted with a pS133-specific or general CREB antibody. (b) Fsk stimulates pS133 phosphorylation of glycosylated and non-glycosylated CREB similarly (n = 6). (c) Inhibition of OGA increases CREB glycosylation levels on phosphorylated and total CREB similarly (n = 4). Neither Fsk nor PUGNAc affected overall CREB glycosylation or pS133 phosphorylation levels, respectively (Supplementary Fig. 7). (d) Complex interplay between MeCP2 glycosylation and phosphorylation. Mass-tagged lysates from neurons treated with GlcN or vehicle were immunoblotted with a pS80-specific or general MeCP2 antibody (n = 6). (e) GlcN increases MeCP2 glycosylation levels predominantly on the pS80 subpopulation. (f) GlcN decreases pS80 levels on the nonglycosylated subpopulation and increases pS80 levels on the glycosylated subpopulation. (g) Reverse yin-yang relationship on MeCP2 under physiological conditions. Mass-tagged nuclear extracts from synchronously depolarized or non-depolarized neurons were immunoblotted with a pS80-specific or general MeCP2 antibody (n = 8). (h) Neuronal depolarization induces pS80 levels on glycosylated MeCP2. (i) Depolarization increases the glycosylation level of pS80 MeCP2, while decreasing that of total MeCP2. UDP ketogalactose 1 was excluded as a control. Data represent mean ± s.e.m. Statistics were analyzed by Student’s _t_-test. * P = 0.0005, ** P = 0.00007 for (b). * P = 0.02, ** P = 0.0007 for (c). * P = 0.04, ** P = 0.008 for (e). * P = 0.01, ** P = 0.04 for (f). * P = 0.01, ** P = 0.001 for (h). * P = 0.04, ** P = 0.001 for (i). Full-length blots are presented in Supplementary Figure 9.
Comment in
- Post-translational modifications: A shift for the O-GlcNAc paradigm.
Kohler JJ. Kohler JJ. Nat Chem Biol. 2010 Sep;6(9):634-5. doi: 10.1038/nchembio.429. Nat Chem Biol. 2010. PMID: 20720545 No abstract available.
Similar articles
- Visualization of O-GlcNAc glycosylation stoichiometry and dynamics using resolvable poly(ethylene glycol) mass tags.
Clark PM, Rexach JE, Hsieh-Wilson LC. Clark PM, et al. Curr Protoc Chem Biol. 2013;5(4):281-302. doi: 10.1002/9780470559277.ch130153. Curr Protoc Chem Biol. 2013. PMID: 24391098 Free PMC article. - Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics.
Khidekel N, Ficarro SB, Clark PM, Bryan MC, Swaney DL, Rexach JE, Sun YE, Coon JJ, Peters EC, Hsieh-Wilson LC. Khidekel N, et al. Nat Chem Biol. 2007 Jun;3(6):339-48. doi: 10.1038/nchembio881. Epub 2007 May 13. Nat Chem Biol. 2007. PMID: 17496889 - Direct Monitoring of Protein O-GlcNAcylation by High-Resolution Native Mass Spectrometry.
Leney AC, Rafie K, van Aalten DMF, Heck AJR. Leney AC, et al. ACS Chem Biol. 2017 Aug 18;12(8):2078-2084. doi: 10.1021/acschembio.7b00371. Epub 2017 Jun 28. ACS Chem Biol. 2017. PMID: 28609614 Free PMC article. - O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar.
Wells L, Hart GW. Wells L, et al. FEBS Lett. 2003 Jul 3;546(1):154-8. doi: 10.1016/s0014-5793(03)00641-0. FEBS Lett. 2003. PMID: 12829252 Review. - Molecular mechanisms of O-GlcNAcylation.
Hurtado-Guerrero R, Dorfmueller HC, van Aalten DM. Hurtado-Guerrero R, et al. Curr Opin Struct Biol. 2008 Oct;18(5):551-7. doi: 10.1016/j.sbi.2008.09.005. Epub 2008 Oct 6. Curr Opin Struct Biol. 2008. PMID: 18822376 Review.
Cited by
- Dynamic O-GlcNAc modification regulates CREB-mediated gene expression and memory formation.
Rexach JE, Clark PM, Mason DE, Neve RL, Peters EC, Hsieh-Wilson LC. Rexach JE, et al. Nat Chem Biol. 2012 Jan 22;8(3):253-61. doi: 10.1038/nchembio.770. Nat Chem Biol. 2012. PMID: 22267118 Free PMC article. - A novel deconvolution method for modeling UDP-N-acetyl-D-glucosamine biosynthetic pathways based on (13)C mass isotopologue profiles under non-steady-state conditions.
Moseley HN, Lane AN, Belshoff AC, Higashi RM, Fan TW. Moseley HN, et al. BMC Biol. 2011 May 31;9:37. doi: 10.1186/1741-7007-9-37. BMC Biol. 2011. PMID: 21627825 Free PMC article. - An enrichment method based on synergistic and reversible covalent interactions for large-scale analysis of glycoproteins.
Xiao H, Chen W, Smeekens JM, Wu R. Xiao H, et al. Nat Commun. 2018 Apr 27;9(1):1692. doi: 10.1038/s41467-018-04081-3. Nat Commun. 2018. PMID: 29703890 Free PMC article. - _O-_GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect.
Wang Y, Liu J, Jin X, Zhang D, Li D, Hao F, Feng Y, Gu S, Meng F, Tian M, Zheng Y, Xin L, Zhang X, Han X, Aravind L, Wei M. Wang Y, et al. Proc Natl Acad Sci U S A. 2017 Dec 26;114(52):13732-13737. doi: 10.1073/pnas.1704145115. Epub 2017 Dec 11. Proc Natl Acad Sci U S A. 2017. PMID: 29229835 Free PMC article. - Elucidating the protein substrate recognition of O-GlcNAc transferase (OGT) toward O-GlcNAcase (OGA) using a GlcNAc electrophilic probe.
Kositzke A, Fan D, Wang A, Li H, Worth M, Jiang J. Kositzke A, et al. Int J Biol Macromol. 2021 Feb 1;169:51-59. doi: 10.1016/j.ijbiomac.2020.12.078. Epub 2020 Dec 18. Int J Biol Macromol. 2021. PMID: 33333092 Free PMC article.
References
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. - PubMed
- Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science. 2008;319:1402–1405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM084724-07/GM/NIGMS NIH HHS/United States
- R01 GM084724/GM/NIGMS NIH HHS/United States
- R01 GM084724-07S1/GM/NIGMS NIH HHS/United States
- F31 NS056525/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases