A spindle-like apparatus guides bacterial chromosome segregation - PubMed (original) (raw)
A spindle-like apparatus guides bacterial chromosome segregation
Jerod L Ptacin et al. Nat Cell Biol. 2010 Aug.
Abstract
Until recently, a dedicated mitotic apparatus that segregates newly replicated chromosomes into daughter cells was believed to be unique to eukaryotic cells. Here we demonstrate that the bacterium Caulobacter crescentus segregates its chromosome using a partitioning (Par) apparatus that has surprising similarities to eukaryotic spindles. We show that the C. crescentus ATPase ParA forms linear polymers in vitro and assembles into a narrow linear structure in vivo. The centromere-binding protein ParB binds to and destabilizes ParA structures in vitro. We propose that this ParB-stimulated ParA depolymerization activity moves the centromere to the opposite cell pole through a burnt bridge Brownian ratchet mechanism. Finally, we identify the pole-specific TipN protein as a new component of the Par system that is required to maintain the directionality of DNA transfer towards the new cell pole. Our results elucidate a bacterial chromosome segregation mechanism that features basic operating principles similar to eukaryotic mitotic machines, including a multivalent protein complex at the centromere that stimulates the dynamic disassembly of polymers to move chromosomes into daughter compartments.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing financial interests.
Figures
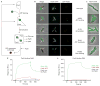
Figure 1
ParA and ParB dynamics in vivo and ParA polymerization in vitro suggest a retracting polymeric ParA structure guides centromere segregation. (a) A retracting ParA structure leads the ParB–parS complex towards the new pole. Time-lapse epifluorescence microscopy of JP110 swarmer cells imaged at 5-min intervals on initiation of S phase. Phase-contrast, ParA–eYFP (green) and CFP–ParB (red) images (top row), or phase and CFP–ParB images (bottom row) are overlaid. The translocating CFP–ParB-bound parS complex is indicated (white arrow). Scale bars, 1 μm. (b) Super-resolution imaging reveals that the retracting ‘cloud’-like ParA in epifluorescence images corresponds to a narrow linear ParA structure. Representative images of JP138 cells at various stages of parS segregation are shown: a diffraction-limited epifluorescence image and corresponding super resolution image of a representative cell (cell A); a cell undergoing parS segregation (Cell B); and a cell after parS segregation is completed (cell C). For the super resolution images, the locations of ParA–eYFP (green) and CFP–ParB (red) molecules are plotted as 2D Gaussians with width defined by the fit error of the single-molecule localizations, and overlaid with the white light cell outline. Scale bars, 1 μm. (c) Purified ParA polymerizes in the presence of ATP in vitro. A representative negative-stain electron micrograph of ParA incubated with ATP is shown (upper panel; scale bar, 100 nm). Higher magnification images (lower panel; scale bar, 20 nm), showing single (lower left) and bundled ParA protofilaments (lower middle and right).
Figure 2
Mutational and biochemical analysis of C. crescentus ParA. (a) Consensus view of the ParA biochemical pathway. Apo–ParA (half-circle) binds ATP (green circle), changes conformation (triangle with green circle), and dimerizes. ParB-stimulated ATP hydrolysis or nucleotide exchange of the ParA dimer (square with green circles) causes release of ADP (red circle) and Pi to reset the cycle. (b) Images of C. crescentus strains expressing merodiploid wild-type or mutant ParA–eYFP. Phase, ParA–eYFP (green) and CFP–ParB (red) are overlaid as shown. White arrows indicate partially translocated ParB foci. Scale bars, 1 μm. (c) Images of E. coli cells expressing wild-type and mutant C. crescentus ParA–eYFP proteins. Phase-contrast and eYFP images (green) are overlaid. Scale bars, 1 μm. (d) ParA requires ATP for interaction with ParB. Surface plasmon resonance (SPR) analysis using immobilized ParB. ParA (500 nM) injected with ATP (green), ADP (red), or no nucleotide (blue) at t = 0, and buffer only (150 s). Response units (R.U.) are plotted versus time (s). (e) ParA requires ATP for non-specific DNA binding. SPR analysis using immobilized non-specific DNA duplex (a scrambled parS sequence). ParA (500nM) injected with ATP (green), ADP (red), or no nucleotide (blue) at t = 0, and buffer only (150 s). Response units (R.U.) are plotted versus time (s).
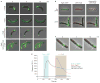
Figure 3
ParB in complex with parS drives the dynamics of ParA structures on DNA. (a) ParB is required for the dynamic movement of ParA structures in vivo. C. crescentus strains in which the only copy of ParB was controlled by the xylose-inducible promoter were cultured in medium with (+ParB) or without (–ParB) xylose, and induced to express ParA–eYFP (green), or ParA–eYFP and mCherry–ParB (+mCherry–ParB) or mCherry–ParBL12A (+mCherry–ParBL12A; red). Phase and eYFP, or phase/eYFP/mCherry images were collected at 5-min intervals and overlaid as shown. Scale bar, 1 μm. (b) ParA localization in E. coli requires ParB and parS for dynamic movement along the nucleoid. The E. coli strains eJP142 (+parS plasmid) and eJP140 (–parS plasmid) were induced to express CFP–ParB (red) and/or ParA–eYFP (green), and phase, eYFP and CFP images were collected and overlaid as shown. The white arrow indicates dynamic ParA–eYFP localization (see c). Scale bar, 1 μm. (c) Time-lapse image series of eJP142 cells showing ParA–eYFP localization dynamics. Cultures were prepared as described in b, and phase, eYFP and CFP images were collected at 5-min intervals and overlaid. The predominant localization of ParA is indicated with a large white arrow, and smaller arrow indicates other localizations. Scale bar, 1 μm. (d) ParB destabilizes a DNA-bound ParA complex in vitro. SPR analysis using an immobilized non-specific 162-nucelotide duplex DNA. ParA (375 nM) was first injected with ATP for 150 s (blue region) followed by buffer only for 150 s. Subsequently, 6His–ParB (1 μM dimer, red trace) or buffer only (green trace) was injected for 6 min (grey region) followed by buffer only. The blue trace shows a flow sequence in which no ParA was injected, followed by 6His–ParB (1 μM dimer), showing negligible non-specific DNA binding by 6His–ParB. The black trace represents a flow sequence lacking ParA and 6His–ParB. Response units (R.U.) are plotted against time (s).
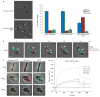
Figure 4
TipN confers new pole-specific directionality to Par-mediated DNA transfer through direct interaction with ParA. (a) Strains lacking tipN show severe parS segregation defects. Synchronized cultures of JP2 (parB::cfp-parB), and of JP138 (vanA::pvan-mCherry-ParB) and JP141 (vanA::pvan-mCherry-ParB, ΔtipN) were induced to express mCherry–ParB and imaged for phase and mCherry or CFP fluorescence after the initiation of S phase. Representative fields of JP138 (upper left panel) and JP141 (lower left panel) are shown. The white arrows indicate partially segregated ParB–parS foci. Scale bar, 1 μm. Mean percentage of cells (right panel) with bipolar ParB foci (blue), unipolar foci (green), or partially translocated foci (red) for JP2, JP138 and JP141. Data are mean ± s.e.m. (n = 3 replicates of >400 cells each). (b) Pauses and reversals of ParB–parS translocation in the absence of tipN. A Δ_tipN_ strain was induced to express ParA–eYFP (green) and mCherry–ParB (red). Synchronized and phase-contrast, eYFP and mCherry fluorescence images were collected at the indicated intervals after the initiation of S phase. A representative Δ_tipN_ cell undergoing parS translocation reversal is shown as phase/eYFP/mCherry overlay. The large white arrows indicate the major ParB-associated ParA localization; smaller arrows indicate other associated ParA structures. Scale bar, 1 μm. (c) Heterologous colocalization assay in E. coli demonstrates that TipN recruits ParA–eYFP into a complex in E. coli. A portion of the Shigella protein IcsA (IcsA507–620) recruits full-length and fragments of C. crescentus TipN to the E. coli cell pole. Full-length TipN (top row), TipNNTD (middle row) or TipNCTD (bottom row) fused to IcsA507–620–mCherry (red) were co-expressed with ParADNA–eYFP (green) in E. coli cells, and imaged for phase contrast, eYFP and mCherry fluorescence. Images are overlaid: phase/mCherry/eYFP (left column), phase/mCherry (middle column), phase/eYFP (right column). fragments. Colocalization is observed only with full-length and TipNCTD (d) Purified ParA and TipNCTD interact directly in vitro. SPR analysis using immobilized TipNCTD. ParA (750 nM) was injected with ATP (green), ADP (red), or no nucleotide (blue), followed by buffer only (150 s). Response units (R.U.) are plotted versus time (s).
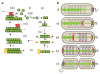
Figure 5
A burnt-bridge Brownian ratchet mechanism for Par-mediated chromosome segregation in C. crescentus. (a) Proposed sequence of molecular interactions during Par-mediated DNA segregation. (i) Apo-ParA (green circle) binds ATP, changes conformation (green box), and (ii) dimerizes, (paired green box). The ParA-ATP homodimer (iii) binds to the nucleoid, or (iv) polymerizes along DNA or in solution (red arrows indicate the direction of polymerization/depolymerization). (v) TipN (yellow circles) may nucleate or stabilize a ParA polymer at the new pole, and (vi) ParA fibres bundle. The ParB–parS complex (red circles/blue parS DNA) (vii) encounters the end of a ParA fibre and binds. ParB stimulates the terminal ParA of a protofilament to release (viii) and the ParB complex ratchets along the end of a retracting ParA structure (blue arrow indicates direction of ParB–parS movement). (b) Diagram showing the proposed mechanism operating within the C. crescentus cell. (i) A C. crescentus swarmer cell. The unreplicated chromosome (brown coil partially associated with ParA) is tethered to the old pole via ParB (red circle) interactions with PopZ (cyan line),. TipN (yellow circle) is positioned at the new pole,. (ii) The ParB–parS complex is released from the pole and duplicated parS (purple line indicates newly replicated DNA) are decorated with ParB, while TipN may effect the formation or stabilization of a ParA fibre structure (green complex) at the new pole. (iii) A ParB–parS complex encounters the ParA structure and binds it. (iv) The ParB–parS complex disassembles the ends of some ParA protofilaments, ratcheting along a receding ParA structure, leaving other ParA filaments behind. (v) The ParB–parS complex is tethered to the polar PopZ complex. The ParA structure reorganizes, and TipN is recruited to the division site to be positioned for subsequent rounds of segregation.
Similar articles
- Bacterial scaffold directs pole-specific centromere segregation.
Ptacin JL, Gahlmann A, Bowman GR, Perez AM, von Diezmann L, Eckart MR, Moerner WE, Shapiro L. Ptacin JL, et al. Proc Natl Acad Sci U S A. 2014 May 13;111(19):E2046-55. doi: 10.1073/pnas.1405188111. Epub 2014 Apr 28. Proc Natl Acad Sci U S A. 2014. PMID: 24778223 Free PMC article. - Caulobacter requires a dedicated mechanism to initiate chromosome segregation.
Toro E, Hong SH, McAdams HH, Shapiro L. Toro E, et al. Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15435-40. doi: 10.1073/pnas.0807448105. Epub 2008 Sep 29. Proc Natl Acad Sci U S A. 2008. PMID: 18824683 Free PMC article. - The Slow Mobility of the ParA Partitioning Protein Underlies Its Steady-State Patterning in Caulobacter.
Surovtsev IV, Lim HC, Jacobs-Wagner C. Surovtsev IV, et al. Biophys J. 2016 Jun 21;110(12):2790-2799. doi: 10.1016/j.bpj.2016.05.014. Biophys J. 2016. PMID: 27332137 Free PMC article. - Bacterial chromosome segregation.
Draper GC, Gober JW. Draper GC, et al. Annu Rev Microbiol. 2002;56:567-97. doi: 10.1146/annurev.micro.56.012302.160729. Epub 2002 Jan 30. Annu Rev Microbiol. 2002. PMID: 12142478 Review. - Resolving chromosome segregation in bacteria.
Hazan R, Ben-Yehuda S. Hazan R, et al. J Mol Microbiol Biotechnol. 2006;11(3-5):126-39. doi: 10.1159/000094049. J Mol Microbiol Biotechnol. 2006. PMID: 16983190 Review.
Cited by
- Uncoupling of nucleotide hydrolysis and polymerization in the ParA protein superfamily disrupts DNA segregation dynamics.
Dobruk-Serkowska A, Caccamo M, Rodríguez-Castañeda F, Wu M, Bryce K, Ng I, Schumacher MA, Barillà D, Hayes F. Dobruk-Serkowska A, et al. J Biol Chem. 2012 Dec 14;287(51):42545-53. doi: 10.1074/jbc.M112.410324. Epub 2012 Oct 23. J Biol Chem. 2012. PMID: 23093445 Free PMC article. - Insight into centromere-binding properties of ParB proteins: a secondary binding motif is essential for bacterial genome maintenance.
Sanchez A, Rech J, Gasc C, Bouet JY. Sanchez A, et al. Nucleic Acids Res. 2013 Mar 1;41(5):3094-103. doi: 10.1093/nar/gkt018. Epub 2013 Jan 23. Nucleic Acids Res. 2013. PMID: 23345617 Free PMC article. - Soj/ParA stalls DNA replication by inhibiting helix formation of the initiator protein DnaA.
Scholefield G, Errington J, Murray H. Scholefield G, et al. EMBO J. 2012 Mar 21;31(6):1542-55. doi: 10.1038/emboj.2012.6. Epub 2012 Jan 27. EMBO J. 2012. PMID: 22286949 Free PMC article. - Image analysis in fluorescence microscopy: bacterial dynamics as a case study.
van Teeffelen S, Shaevitz JW, Gitai Z. van Teeffelen S, et al. Bioessays. 2012 May;34(5):427-36. doi: 10.1002/bies.201100148. Epub 2012 Mar 13. Bioessays. 2012. PMID: 22415868 Free PMC article. Review. - Poles apart: prokaryotic polar organelles and their spatial regulation.
Kirkpatrick CL, Viollier PH. Kirkpatrick CL, et al. Cold Spring Harb Perspect Biol. 2011 Mar 1;3(3):a006809. doi: 10.1101/cshperspect.a006809. Cold Spring Harb Perspect Biol. 2011. PMID: 21084387 Free PMC article. Review.
References
- Lam H, Schofield WB, Jacobs-Wagner C. A landmark protein essential for establishing and perpetuating the polarity of a bacterial cell. Cell. 2006;124:1011–1023. - PubMed
- Huitema E, Pritchard S, Matteson D, Radhakrishnan SK, Viollier PH. Bacterial birth scar proteins mark future flagellum assembly site. Cell. 2006;124:1025–1037. - PubMed
- Gerdes K, Moller-Jensen J, Bugge Jensen R. Plasmid and chromosome partitioning: surprises from phylogeny. Mol Microbiol. 2000;37:455–466. - PubMed
- Mohl DA, Easter J, Jr, Gober JW. The chromosome partitioning protein, ParB, is required for cytokinesis in Caulobacter crescentus. Mol Microbiol. 2001;42:741–755. - PubMed
- Mohl DA, Gober JW. Cell cycle-dependent polar localization of chromosome partitioning proteins in Caulobacter crescentus. Cell. 1997;88:675–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01GM086196-2/GM/NIGMS NIH HHS/United States
- R01 GM086196-04/GM/NIGMS NIH HHS/United States
- R01 GM51426/GM/NIGMS NIH HHS/United States
- F32GM088966-1/GM/NIGMS NIH HHS/United States
- R01 GM086196/GM/NIGMS NIH HHS/United States
- R01 GM051426/GM/NIGMS NIH HHS/United States
- R01 GM086196-03/GM/NIGMS NIH HHS/United States
- F32 GM088966/GM/NIGMS NIH HHS/United States
- R24 GM073011-04/GM/NIGMS NIH HHS/United States
- R24 GM073011/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources