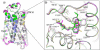Conserved binding mode of human beta2 adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography - PubMed (original) (raw)
Conserved binding mode of human beta2 adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography
Daniel Wacker et al. J Am Chem Soc. 2010.
Abstract
G protein-coupled receptors (GPCRs) represent a large fraction of current pharmaceutical targets, and of the GPCRs, the beta(2) adrenergic receptor (beta(2)AR) is one of the most extensively studied. Previously, the X-ray crystal structure of beta(2)AR has been determined in complex with two partial inverse agonists, but the global impact of additional ligands on the structure or local impacts on the binding site are not well-understood. To assess the extent of such ligand-induced conformational differences, we determined the crystal structures of a previously described engineered beta(2)AR construct in complex with two inverse agonists: ICI 118,551 (2.8 A), a recently described compound (2.8 A) (Kolb et al, 2009), and the antagonist alprenolol (3.1 A). The structures show the same overall fold observed for the previous beta(2)AR structures and demonstrate that the ligand binding site can accommodate compounds of different chemical and pharmacological properties with only minor local structural rearrangements. All three compounds contain a hydroxy-amine motif that establishes a conserved hydrogen bond network with the receptor and chemically diverse aromatic moieties that form distinct interactions with beta(2)AR. Furthermore, receptor ligand cross-docking experiments revealed that a single beta(2)AR complex can be suitable for docking of a range of antagonists and inverse agonists but also indicate that additional ligand-receptor structures may be useful to further improve performance for in-silico docking or lead-optimization in drug design.
Figures
Figure 1
Structural comparison of the ligand binding sites in the (a) ICIβ2AR-t4l, (b) 2β2AR-t4l and (c) Alpβ2AR-t4l crystal structures. The ligands 1 (ICI 118,551), 2 and 3 (alprenolol) are colored in darker shades of orange, green and blue, respectively, while residues around the binding site are colored in lighter shades and labeled. Hydrogen bonds are depicted as black dotted lines. Chemical structures of compounds are shown in boxes.
Figure 2
Conserved overall fold of the ICIβ2AR-t4l, 2β2AR-t4l and Alpβ2AR-t4l structures compared to Timβ2AR-t4l and Carβ2AR-t4l. (a) Superimposition of all β2AR-t4l crystal structures determined to date (t4l omitted); ICIβ2AR-t4l (yellow), 2β2AR-t4l (green), Alpβ2AR-t4l (blue), Timβ2AR-t4l (magenta) and Carβ2AR-t4l (grey) (b) Close view of the ligand binding site showing the conserved binding of the hydroxy-amine motif and the differences in the aromatic system moieties. Compounds are shown as sticks and surrounding residue side chains are shown as lines. Superscripts indicate the Ballesteros-Weinstein numbering convention.
Similar articles
- Ligand-specific interactions modulate kinetic, energetic, and mechanical properties of the human β2 adrenergic receptor.
Zocher M, Fung JJ, Kobilka BK, Müller DJ. Zocher M, et al. Structure. 2012 Aug 8;20(8):1391-402. doi: 10.1016/j.str.2012.05.010. Epub 2012 Jun 28. Structure. 2012. PMID: 22748765 Free PMC article. - Structure-Based Prediction of G-Protein-Coupled Receptor Ligand Function: A β-Adrenoceptor Case Study.
Kooistra AJ, Leurs R, de Esch IJ, de Graaf C. Kooistra AJ, et al. J Chem Inf Model. 2015 May 26;55(5):1045-61. doi: 10.1021/acs.jcim.5b00066. Epub 2015 May 1. J Chem Inf Model. 2015. PMID: 25848966 - Structural features of β2 adrenergic receptor: crystal structures and beyond.
Bang I, Choi HJ. Bang I, et al. Mol Cells. 2015;38(2):105-11. doi: 10.14348/molcells.2015.2301. Epub 2014 Dec 24. Mol Cells. 2015. PMID: 25537861 Free PMC article. Review. - Modeling G protein-coupled receptors in complex with biased agonists.
Costanzi S. Costanzi S. Trends Pharmacol Sci. 2014 Jun;35(6):277-83. doi: 10.1016/j.tips.2014.04.004. Epub 2014 May 2. Trends Pharmacol Sci. 2014. PMID: 24793542 Review.
Cited by
- Agonist binding by the β2-adrenergic receptor: an effect of receptor conformation on ligand association-dissociation characteristics.
Plazinska A, Plazinski W, Jozwiak K. Plazinska A, et al. Eur Biophys J. 2015 Apr;44(3):149-63. doi: 10.1007/s00249-015-1010-4. Epub 2015 Mar 1. Eur Biophys J. 2015. PMID: 25726162 Free PMC article. - Detection of G protein-selective G protein-coupled receptor (GPCR) conformations in live cells.
Malik RU, Ritt M, DeVree BT, Neubig RR, Sunahara RK, Sivaramakrishnan S. Malik RU, et al. J Biol Chem. 2013 Jun 14;288(24):17167-78. doi: 10.1074/jbc.M113.464065. Epub 2013 Apr 29. J Biol Chem. 2013. PMID: 23629648 Free PMC article. - Function-specific virtual screening for GPCR ligands using a combined scoring method.
Kooistra AJ, Vischer HF, McNaught-Flores D, Leurs R, de Esch IJ, de Graaf C. Kooistra AJ, et al. Sci Rep. 2016 Jun 24;6:28288. doi: 10.1038/srep28288. Sci Rep. 2016. PMID: 27339552 Free PMC article. - Compound activity prediction using models of binding pockets or ligand properties in 3D.
Kufareva I, Chen YC, Ilatovskiy AV, Abagyan R. Kufareva I, et al. Curr Top Med Chem. 2012;12(17):1869-82. doi: 10.2174/156802612804547335. Curr Top Med Chem. 2012. PMID: 23116466 Free PMC article. Review. - Characterizing and predicting the functional and conformational diversity of seven-transmembrane proteins.
Abrol R, Kim SK, Bray JK, Griffith AR, Goddard WA 3rd. Abrol R, et al. Methods. 2011 Dec;55(4):405-14. doi: 10.1016/j.ymeth.2011.12.005. Epub 2011 Dec 17. Methods. 2011. PMID: 22197575 Free PMC article.
References
- Audet M, Bouvier M. Nat Chem Biol. 2008;4:397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM074961/GM/NIGMS NIH HHS/United States
- P50 GM073197-06/GM/NIGMS NIH HHS/United States
- GM073197/GM/NIGMS NIH HHS/United States
- P50 GM073197/GM/NIGMS NIH HHS/United States
- GM075915/GM/NIGMS NIH HHS/United States
- U54 GM074961/GM/NIGMS NIH HHS/United States
- R01 GM075915/GM/NIGMS NIH HHS/United States
- Y1-GM-1104/GM/NIGMS NIH HHS/United States
- U54 GM074961-050001/GM/NIGMS NIH HHS/United States
- Y1-CO-1020/CO/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials