A diacylglycerol-dependent signaling pathway contributes to regulation of antibacterial autophagy - PubMed (original) (raw)
A diacylglycerol-dependent signaling pathway contributes to regulation of antibacterial autophagy
Shahab Shahnazari et al. Cell Host Microbe. 2010.
Abstract
Autophagy mediates the degradation of cytoplasmic contents in the lysosome and plays a significant role in immunity. Lipid second messengers have previously been implicated in the regulation of autophagy. Here, we demonstrate a signaling role for diacylglycerol (DAG) in antibacterial autophagy. DAG production was necessary for efficient autophagy of Salmonella, and its localization to bacteria-containing phagosomes preceded autophagy. The actions of phospholipase D and phosphatidic acid phosphatase were required for DAG generation and autophagy. Furthermore, the DAG-responsive delta isoform of protein kinase C was required, as were its downstream targets JNK and NADPH oxidase. Previous studies have revealed a role for the ubiquitin-binding adaptor molecules p62 and NDP52 in autophagy of S. Typhimurium. We observed bacteria-containing autophagosomes colocalizing individually with either DAG or ubiquitinated proteins, indicating that both signals can act independently to promote antibacterial autophagy. These findings reveal an important role for DAG-mediated PKC function in mammalian antibacterial autophagy.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
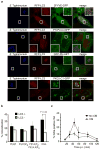
Figure 1. DAG colocalizes with bacteria-containing autophagosomes. (a)
HeLa cells were co-transfected with RFP-LC3 and either 2FYVE-GFP (PI(3)P probe), PLCδ-PH-GFP (PI(4,5)P2 probe), GFP-PH-AKT (PI(3,5)P2 and PI(3,4,5)P3 probe) or PKCδ-C1-GFP (DAG probe). Cells were infected with wild-type S. Typhimurium, fixed at 1 h p.i. and immunostained for S. Typhimurium. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (b) The percentage of RFP-LC3+ or RFP-LC3− bacteria colocalizing with the lipid probes in a was determined by fluorescence microscopy. (c) HeLa cells were transfected with PKCδ-C1-GFP and infected as in a. Cells were treated with or without chloramphenicol (CM, 200 μg/mL) at 10 min p.i. for the remainder of the infection. Cells were fixed at the indicated time-points and immunostained for S. Typhimurium. The percentage of PKCδ-C1-GFP+ bacteria was enumerated by fluorescence microscopy. Data represent the mean ± standard error (s.e.m.) for three independent experiments.
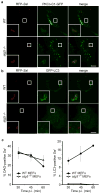
Figure 2. DAG production on SCVs is independent of autophagy. (a-b)
Wild-type (WT) and autophagy-deficient (atg5 −/−) MEFs were transfected with either PKCδ-C1-GFP (a) or GFP-LC3 (b). Cells were infected with S. Typhimurium expressing mRFP (RFP-Sal). Cells were fixed at 45 min a or 1 h b p.i. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (c) WT and _atg5_−/− MEFs were transfected and infected as in a-b and fixed at the indicated time-points. PKCδ-C1-GFP+ or GFP-LC3+ RFP-Sal were determined by fluorescence microscopy. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
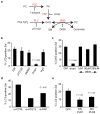
Figure 3. Autophagy of S. Typhimurium requires phosphatidic acid phosphatase and phospholipase D. (a)
Pathways for the production of DAG. PC (phosphatidylcholine), PLD (phopholipase D), PA (phosphatidic acid), PAP (phosphatidic acid phosphatase), propr. (propranolol hydrochloride), PLC (phospholipase C), SMS (sphingomyelin synthase), SM (sphingomyelin). (b) HeLa cells were transfected with GFP-LC3 and infected with RFP-Sal. Cells were treated with growth medium (GM), U73122(10 μM), DMSO, propranolol hydrochloride (propr., 250 μM) or H2O at 10 min p.i. for the remainder of the infection, and fixed at 1 h p.i. (c) HeLa cells were transfected with PKCδ-C1-GFP and infected with RFP-Sal. Cells were treated with GM, propranolol hydrochloride (propr., 250 μM) or D609 at the indicated concentrations at 10 min p.i. for the remainder of the infection, and fixed at 45 min p.i.. (d) HeLa cells were co-transfected with GFP-LC3 and either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12) or PAP2B (si-PAP). Cells were infected with RFP-Sal and fixed at 1 h p.i. (e) HeLa cells were transfected with RFP-LC3 and either GFP, HA-PLD1 DN (dominant negative) or HA-PLD2 DN. Cells were infected with S. Typhimurium, fixed at 1 h p.i. and immunostained for S. Typhimurium and HA tag. The percentage of DAG+ or LC3+ S. Typhimurium was determined by fluorescence microscopy. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
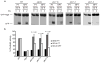
Figure 4. Autophagy of S. Typhimurium requires protein kinase C. (a-b)
HeLa cells were co-transfected with GFP-LC3 and either control siRNA (si-CTRL) or siRNA specifically targeting Atg12 (si-ATG12), PKCδ (si-PKCδ) or PKCα (si-PKCα). Cells were infected with wild-type RFP-Sal and fixed at 1 h p.i. Representative confocal z-slices are shown a. The inner panels represent a higher magnification of the boxed areas. Size bar represents 10 μm. The percentage of GFP-LC3+ bacteria was determined by fluorescence microscopy b. (c) WT and PKCδ-deficient (PKCδ−/−) MEFs were transfected with GFP-LC3 and/or a PKCδ expression plasmid. Cells were infected with RFP-Sal and fixed at 1 h p.i. The percentage of GFP-LC3+ bacteria was determined by fluorescence microscopy. (d) WT and PKCδ-deficient (PKCδ −/−) MEFs were infected with wild-type S. Typhimurim and lysed at the indicated time points. The number of bacteria was determined by the number of colonies formed on agar plates. Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
Figure 5. Pkc1 is required for starvation-induced autophagy in yeast. (a)
GFP-Atg8 processing was monitored in wild-type (TN124), _atg1_Δ (TYY127), pkc1ts, pkc1-3 and pkc1-4 cells expressing GFP-Atg8. Cells were grown in SD-N medium at the permissive (PT, 24°C) or non-permissive (NPT, 38 C) temperature. Full-length GFP-Atg8 and free GFP were detected by Western blotting using anti-YFP antibodies. (b) Autophagic activity was determined by obtaining extracts from wild-type (TN124), _atg1_Δ (TYY127), pkc1ts, pkc1-3 and pkc1-4 S. cerevisiae expressing Pho8Δ60 and analyzed for Pho8Δ60-dependent alkaline phosphatase activity. Synthetic defined media lacking nitrogen (SD-N). Data represent the mean ± standard error (s.e.m.) for at least three independent experiments.
Figure 6. DAG and p62 act in independent signaling pathways for anti-bacterial autophagy. (a)
HeLa cells were co-transfected with PKCδ-C1-GFP and RFP-LC3. Cells were infected with wild-type S. Typhimurium, fixed at 45 min p.i. and immunostained for S. Typhimurium and mono- and polyubiquitinated proteins. Representative confocal z-slices are shown. The inner panels represent a higher magnification of the boxed areas. Size bar, 10 μm. (b) HeLa cells were transfected with PKCδ-C1-GFP, infected with wild-type S. Typhimurium and fixed at 45 and 60 min p.i.. Cells were immunostained for ubiquitinated protein and bacteria as in a. DAG+ bacteria were scored for colocalization with ubiquitin. (c) HeLa cells were transfected with PKCδ-C1-GFP, infected with wild-type S. Typhimurium, fixed at 45 min p.i. and immunostained for S. Typhimurium and p62. p62 colocalization was quantified for DAG+ and DAG− bacteria (d) HeLa cells were co-transfected with GFP-LC3 and either control siRNA (si-CTRL) or siRNA specifically targeting PKCδ (si-PKCδ) PAP (si-PAP) or p62 (si-p62). Cells were infected with wild-type S. Typhimurium and treated with 15 μM rottlerin where indicated. Cells were fixed at 1 h p.i. and quantified for colocalization with GFP-LC3. Data represent the mean ± standard error (s.e.m.) for three independent experiments.
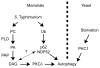
Figure 7. Two signals target S. Typhimurium to the autophagy pathway
Model depicting the dual pathways by which S. Typhimurium is targeted by autophagy in mammals and the conserved role for PKC in the regulation of this process in both mammals and yeast. Data is consistent with two independent pathways for induction of antibacterial autophagy in mammalian cells (DAG and ubiquitin pathways). Dashed line and question mark between pathways represents the possibility that the two pathways may interact with each other.
Comment in
- Autophagy employs a new DAGger against bacteria.
Lafont F. Lafont F. Cell Host Microbe. 2010 Aug 19;8(2):129-30. doi: 10.1016/j.chom.2010.07.009. Cell Host Microbe. 2010. PMID: 20709289
Similar articles
- Signal transduction in vascular smooth muscle: diacylglycerol second messengers and PKC action.
Lee MW, Severson DL. Lee MW, et al. Am J Physiol. 1994 Sep;267(3 Pt 1):C659-78. doi: 10.1152/ajpcell.1994.267.3.C659. Am J Physiol. 1994. PMID: 7943196 Review. - Intracellular Salmonella induces aggrephagy of host endomembranes in persistent infections.
López-Montero N, Ramos-Marquès E, Risco C, García-Del Portillo F. López-Montero N, et al. Autophagy. 2016 Oct 2;12(10):1886-1901. doi: 10.1080/15548627.2016.1208888. Epub 2016 Aug 2. Autophagy. 2016. PMID: 27485662 Free PMC article. - Muscarinic receptor regulation of protein kinase C distribution and phosphatidylcholine hydrolysis.
Brown JH, Trilivas I, Martinson EA. Brown JH, et al. Symp Soc Exp Biol. 1990;44:147-56. Symp Soc Exp Biol. 1990. PMID: 2130511 - A phospholipase D-mediated pathway for generating diacylglycerol in nuclei from Madin-Darby canine kidney cells.
Balboa MA, Balsinde J, Dennis EA, Insel PA. Balboa MA, et al. J Biol Chem. 1995 May 19;270(20):11738-40. doi: 10.1074/jbc.270.20.11738. J Biol Chem. 1995. PMID: 7538121 - Spatiotemporal dynamics of lipid signaling: protein kinase C as a paradigm.
Gallegos LL, Newton AC. Gallegos LL, et al. IUBMB Life. 2008 Dec;60(12):782-9. doi: 10.1002/iub.122. IUBMB Life. 2008. PMID: 18720411 Free PMC article. Review.
Cited by
- Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion.
Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, Randow F. Thurston TL, et al. Nature. 2012 Jan 15;482(7385):414-8. doi: 10.1038/nature10744. Nature. 2012. PMID: 22246324 Free PMC article. - Autophagy in immunity: implications in etiology of autoimmune/autoinflammatory diseases.
Zhou XJ, Zhang H. Zhou XJ, et al. Autophagy. 2012 Sep;8(9):1286-99. doi: 10.4161/auto.21212. Epub 2012 Aug 14. Autophagy. 2012. PMID: 22878595 Free PMC article. Review. - Antigen Processing for MHC Class II Presentation via Autophagy.
Münz C. Münz C. Front Immunol. 2012 Feb 2;3:9. doi: 10.3389/fimmu.2012.00009. eCollection 2012. Front Immunol. 2012. PMID: 22566895 Free PMC article. - How ubiquitination and autophagy participate in the regulation of the cell response to bacterial infection.
Dupont N, Temime-Smaali N, Lafont F. Dupont N, et al. Biol Cell. 2010 Dec;102(12):621-34. doi: 10.1042/BC20100101. Biol Cell. 2010. PMID: 21077843 Free PMC article. Review. - Autophagy in immunity and inflammation.
Levine B, Mizushima N, Virgin HW. Levine B, et al. Nature. 2011 Jan 20;469(7330):323-35. doi: 10.1038/nature09782. Nature. 2011. PMID: 21248839 Free PMC article. Review.
References
- Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281:11374–11383. - PubMed
- Brumell JH, Grinstein S. Salmonella redirects phagosomal maturation. Curr Opin Microbiol. 2004;7:78–84. - PubMed
- Brumell JH, Rosenberger CM, Gotto GT, Marcus SL, Finlay BB. SifA permits survival and replication of Salmonella typhimurium in murine macrophages. Cell Microbiol. 2001;3:75–84. - PubMed
- Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous