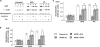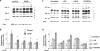Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice - PubMed (original) (raw)
Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice
Susan A Marsh et al. Amino Acids. 2011 Mar.
Abstract
Patients with diabetes have a much greater risk of developing heart failure than non-diabetic patients, particularly in response to an additional hemodynamic stress such as hypertension or infarction. Previous studies have shown that increased glucose metabolism via the hexosamine biosynthesis pathway (HBP) and associated increase in O-linked-β-N-acetylglucosamine (O-GlcNAc) levels on proteins contributed to the adverse effects of diabetes on the heart. Therefore, in this study we tested the hypothesis that diabetes leads to impaired cardiomyocyte hypertrophic and cell signaling pathways due to increased HBP flux and O-GlcNAc modification on proteins. Cardiomyocytes isolated from type 2 diabetic db/db mice and non-diabetic controls were treated with 1 μM ANG angiotensin II (ANG) and 10 μM phenylephrine (PE) for 24 h. Activation of hypertrophic and cell signaling pathways was determined by assessing protein expression levels of atrial natriuretic peptide (ANP), α-sarcomeric actin, p53, Bax and Bcl-2 and phosphorylation of p38, ERK and Akt. ANG II and PE significantly increased levels of ANP and α-actin and phosphorylation of p38 and ERK in the non-diabetic but not in the diabetic group; phosphorylation of Akt was unchanged irrespective of group or treatment. Constitutive Bcl-2 levels were lower in diabetic hearts, while there was no difference in p53 and Bax. Activation of the HBP and increased protein O-GlcNAcylation in non-diabetic cardiomyocytes exhibited a significantly decreased hypertrophic signaling response to ANG or PE compared to control cells. Inhibition of the HBP partially restored the hypertrophic signaling response of diabetic cardiomyocytes. These results suggest that activation of the HBP and protein O-GlcNAcylation modulates hypertrophic and cell signaling pathways in type 2 diabetes.
Figures
Fig. 1
Expression of a ANP, b α-actin and c TRPC1 protein in cardiomyocytes isolated from non-diabetic control (control) and diabetic (db/db) hearts following 24 h treatment with ANG (1 μM) or PE (10 μM). Upper panels are representative immunoblots and the lower panels are mean densitometric data from three individual experiments normalized to calsequestrin. *P < 0.05 versus control untreated group
Fig. 2
Expression of phosphorylated (P) and total (T) a p38, b ERK and c Akt protein in cardiomyocytes isolated from non-diabetic control (control) and diabetic (db/db) hearts following 24 h treatment with ANG (1 μM) or PE (10 μM). Upper panels are representative immunoblots and the lower panels are mean densitometric data from three individual experiments of phosphorylated proteins normalized to their respective total protein. *P < 0.05 versus control untreated group
Fig. 3
a Immunoblots for ANP, α-actin and calsequestrin in untreated cardiomyocytes and cardiomyocytes following 24 h treatment with ANG (1 μM) or PE (10 μM). Cardiomyocytes isolated from db/db animals (d) were also treated with the GFAT inhibitors azaserine (5 μM) or Don (20 μM). Mean densitometric data for b ANP and c α-actin expression normalized to calsequestrin from three individual experiments. Data presented as mean ± SE of five individual experiments. *P < 0.05 versus db/db group induced with ANG or PE
Fig. 4
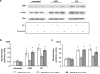
a Representative anti-_O_-GlcNAc immunoblots of whole heart homogenates from non-diabetic mice (control; n = 3) and three diabetic (db/db; n = 3) mice; b mean intensity of _O_-GlcNAc proteins determined by densitometric analysis with levels normalized to calsequestrin for bands 1–5 as indicated; c representative anti-_O_-GlcNAc immunoblots from cardiomyocytes treated with high glucose (25 mM; +HG), glucosamine (5 mM; +GlcN) or PUGNAc (100 μM; +PUGNAc) for 24 h; d mean intensity of _O_-GlcNAc proteins determined by densitometric analysis with levels normalized to calsequestrin for all bands and bands 1–5 as indicated. *P < 0.05 versus control or untreated
Fig. 5
a Immunoblots for ANP, α-actin and calsequestrin in control non-diabetic cardiomyocytes following 24 h treatment with ANG (1 μM) or PE (10 μM) in the presence of high glucose (25 mM; +HG), glucosamine (5 mM; +GlcN) or PUGNAc (100 μM; +PUGNAc). Mean densitometric data for b ANP and c α-actin expression normalized to calsequestrin from three individual experiments. *P < 0.05 versus 5 mM glucose
Fig. 6
a Immunoblots for ANP, α-actin and calsequestrin in control non-diabetic cardiomyocytes following 24 h treatment with ANG (1 μM) or PE (10 μM) in the presence of normal glucose (5 mM), high glucose (25 mM; HG) or
D
-mannitol (25 mM). Mean densitometric data for b ANP and c α-actin expression normalized to calsequestrin from three individual experiments. *P < 0.05 versus 5 mM glucose
Fig. 7
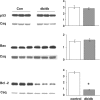
Expression levels and mean densitometric data of pro-apoptotic proteins p53 and Bax, and the anti-apoptotic protein Bcl-2 in the hearts of non-diabetic (Con) and diabetic (db/db) mouse whole heart homogenates. *P < 0.05 versus Con
Similar articles
- First characterization of glucose flux through the hexosamine biosynthesis pathway (HBP) in ex vivo mouse heart.
Olson AK, Bouchard B, Zhu WZ, Chatham JC, Des Rosiers C. Olson AK, et al. J Biol Chem. 2020 Feb 14;295(7):2018-2033. doi: 10.1074/jbc.RA119.010565. Epub 2020 Jan 8. J Biol Chem. 2020. PMID: 31915250 Free PMC article. - Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system.
Fülöp N, Marchase RB, Chatham JC. Fülöp N, et al. Cardiovasc Res. 2007 Jan 15;73(2):288-97. doi: 10.1016/j.cardiores.2006.07.018. Epub 2006 Jul 29. Cardiovasc Res. 2007. PMID: 16970929 Free PMC article. Review. - Hexosamine biosynthetic pathway promotes the antiviral activity of SAMHD1 by enhancing O-GlcNAc transferase-mediated protein O-GlcNAcylation.
Hu J, Gao Q, Yang Y, Xia J, Zhang W, Chen Y, Zhou Z, Chang L, Hu Y, Zhou H, Liang L, Li X, Long Q, Wang K, Huang A, Tang N. Hu J, et al. Theranostics. 2021 Jan 1;11(2):805-823. doi: 10.7150/thno.50230. eCollection 2021. Theranostics. 2021. PMID: 33391506 Free PMC article. - Hyperglycemia-mediated activation of the hexosamine biosynthetic pathway results in myocardial apoptosis.
Rajamani U, Essop MF. Rajamani U, et al. Am J Physiol Cell Physiol. 2010 Jul;299(1):C139-47. doi: 10.1152/ajpcell.00020.2010. Epub 2010 Apr 21. Am J Physiol Cell Physiol. 2010. PMID: 20410435 - Insights into the role of maladaptive hexosamine biosynthesis and O-GlcNAcylation in development of diabetic cardiac complications.
Qin CX, Sleaby R, Davidoff AJ, Bell JR, De Blasio MJ, Delbridge LM, Chatham JC, Ritchie RH. Qin CX, et al. Pharmacol Res. 2017 Feb;116:45-56. doi: 10.1016/j.phrs.2016.12.016. Epub 2016 Dec 14. Pharmacol Res. 2017. PMID: 27988387 Review.
Cited by
- O-GlcNAcylation of 8-Oxoguanine DNA Glycosylase (Ogg1) Impairs Oxidative Mitochondrial DNA Lesion Repair in Diabetic Hearts.
Cividini F, Scott BT, Dai A, Han W, Suarez J, Diaz-Juarez J, Diemer T, Casteel DE, Dillmann WH. Cividini F, et al. J Biol Chem. 2016 Dec 16;291(51):26515-26528. doi: 10.1074/jbc.M116.754481. Epub 2016 Nov 5. J Biol Chem. 2016. PMID: 27816939 Free PMC article. - Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation.
Laczy B, Fülöp N, Onay-Besikci A, Des Rosiers C, Chatham JC. Laczy B, et al. PLoS One. 2011 Apr 11;6(4):e18417. doi: 10.1371/journal.pone.0018417. PLoS One. 2011. PMID: 21494549 Free PMC article. - Stromal interaction molecule 1 is essential for normal cardiac homeostasis through modulation of ER and mitochondrial function.
Collins HE, He L, Zou L, Qu J, Zhou L, Litovsky SH, Yang Q, Young ME, Marchase RB, Chatham JC. Collins HE, et al. Am J Physiol Heart Circ Physiol. 2014 Apr 15;306(8):H1231-9. doi: 10.1152/ajpheart.00075.2014. Epub 2014 Feb 28. Am J Physiol Heart Circ Physiol. 2014. PMID: 24585777 Free PMC article. - Protein O-GlcNAcylation and cardiovascular (patho)physiology.
Marsh SA, Collins HE, Chatham JC. Marsh SA, et al. J Biol Chem. 2014 Dec 12;289(50):34449-56. doi: 10.1074/jbc.R114.585984. Epub 2014 Oct 21. J Biol Chem. 2014. PMID: 25336635 Free PMC article. Review. - Functional O-GlcNAc modifications: implications in molecular regulation and pathophysiology.
Vaidyanathan K, Durning S, Wells L. Vaidyanathan K, et al. Crit Rev Biochem Mol Biol. 2014 Mar-Apr;49(2):140-163. doi: 10.3109/10409238.2014.884535. Epub 2014 Feb 14. Crit Rev Biochem Mol Biol. 2014. PMID: 24524620 Free PMC article. Review.
References
- Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. - PubMed
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. - PubMed
- Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia–reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol. 2007;292:C178–C187. - PubMed
- Chatham JC, Forder JR, McNeill JH, editors. The Heart in Diabetes. Kluwer Academic Publishers; Norwell: 1996.
Publication types
MeSH terms
Substances
Grants and funding
- P50 HL077100-04/HL/NHLBI NIH HHS/United States
- R01 HL067464/HL/NHLBI NIH HHS/United States
- HL-101192/HL/NHLBI NIH HHS/United States
- R01 HL067464-06/HL/NHLBI NIH HHS/United States
- R01 HL101192/HL/NHLBI NIH HHS/United States
- HL-67464/HL/NHLBI NIH HHS/United States
- P50 HL077100/HL/NHLBI NIH HHS/United States
- HL-77100/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous