Apoptosis-promoted tumorigenesis: gamma-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death - PubMed (original) (raw)
Apoptosis-promoted tumorigenesis: gamma-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death
Ewa M Michalak et al. Genes Dev. 2010.
Abstract
Although tumor development requires impaired apoptosis, we describe a novel paradigm of apoptosis-dependent tumorigenesis. Because DNA damage triggers apoptosis through p53-mediated induction of BH3-only proteins Puma and Noxa, we explored their roles in gamma-radiation-induced thymic lymphomagenesis. Surprisingly, whereas Noxa loss accelerated it, Puma loss ablated tumorigenesis. Tumor suppression by Puma deficiency reflected its protection of leukocytes from gamma-irradiation-induced death, because their glucocorticoid-mediated decimation in Puma-deficient mice activated cycling of stem/progenitor cells and restored thymic lymphomagenesis. Our demonstration that cycles of cell attrition and repopulation by stem/progenitor cells can drive tumorigenesis has parallels in human cancers, such as therapy-induced malignancies.
Figures
Figure 1.
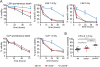
Noxa deficiency enhances γ-irradiation-induced thymic lymphoma development. (A) Kaplan-Meier curves showing percentage survival of tumor-free mice of the indicated genotypes exposed to four weekly doses of γ-irradiation (1.5 Gy). Thymic lymphomas arose with higher incidence and significantly (P < 0.0005) earlier in _noxa_−/− mice (n = 16) than wild-type mice (n = 23). Differences in tumor onset between wild-type and noxa+/− mice (n = 21), and between _noxa_−/− and p53+/− mice (n = 11) were not significant (P = 0.064 and P = 0.76, respectively). (B) Western blot analysis of p19Arf and β-actin (loading control) in unselected thymic lymphomas of the indicated genotypes. p53+/− thymic lymphomas and _p53_−/− mouse embryonic fibroblasts (MEF) provided positive controls for p19Arf overexpression. Protein size standards in kilodaltons are indicated. (C) Total leukocyte cellularity of the bone marrow and thymus from wild-type and _noxa_−/− mice that had received 1.5 Gy γ-irradiation on days 0 and 7. Mean ± SEM, n = 3–7 for each genotype and day of analysis.
Figure 2.
Sensitivity of leukocyte populations from Noxa- or Puma-deficient mice to γ-irradiation-induced or spontaneous apoptosis. (A) Sensitivity in vitro of the indicated bone marrow cell subsets to γ-irradiation or spontaneous death (no cytokine support). LSK stem/progenitor cells and CLPs isolated from the marrow of wild-type, _noxa_−/−, and _puma_−/− mice were left untreated or were γ-irradiated (1.5 or 5 Gy), and were then cultured in vitro, and viability was assessed by Annexin V/PI staining and FACS analysis. Mean ± SEM, n = 3-4. (*) P < 0.05; (**) P < 0.01; (***) P < 0.001. (B) Bone marrow cells from wild-type, _noxa_−/−, and _puma_−/− mice were γ-irradiated (1.5 Gy) and transplanted into γ-irradiated wild-type recipients, and macroscopic spleen colonies (CFU-S) were counted 12 d later. Mean ± SEM, n = 6–12 donor mice. (*) P < 0.05; (**) P < 0.01; (***) P < 0.001. CFU-S numbers from untreated marrow cells of these genotypes were comparable (Supplemental Fig. 5D).
Figure 3.
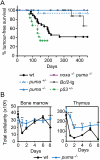
Loss of Puma prevents γ-irradiation-induced thymic lymphoma development. (A) Kaplan-Meier curves showing percentage tumor-free survival of mice exposed to four weekly doses of γ-irradiation (1.5 Gy). Only one out of 15 puma+/− mice developed a thymic lymphoma (CD4+8+) after 137 d; regenotyping confirmed puma heterozygosity in this animal. Only one out of 19 _vav_-_bcl_-2 transgenic mice developed a thymic lymphoma (after 349 d); all other deaths reflected systemic lupus erythematosus (SLE)-like autoimmune disease or follicular B-cell lymphoma—diseases common in these animals (Egle et al. 2004a). No thymic lymphomas arose in _puma_−/− (n = 18) or _noxa_−/−_puma_−/− (n = 16) mice. Thymic lymphomas arose faster in p53+/− mice (n = 11) than wild-type mice (n = 28; P < 0.02) and faster in wild-type mice than all other genotypes shown (P < 0.004). (B) Total leukocyte cellularity of the bone marrow and thymus from wild-type and _puma_−/− mice following γ-irradiation (1.5 Gy) on days 0 and 7. Mean ± SEM, n = 2–5. (**) P < 0.01; (***) P < 0.001. Data on bone marrow and thymus cellularity for wild-type mice are the same as those shown in Figure 1C—as wild-type, _noxa_−/−, and _puma_−/− mice were studied in parallel—but the data from _noxa_−/− and _puma_−/− mice are presented in two separate figures (B and Fig. 1C) and are described in separate parts of the text for the sake of clarity.
Figure 4.
Leukocyte depletion by dexamethasone restores γ-irradiation-induced thymic lymphoma development in Puma-deficient mice. (A) Total cellularity of the bone marrow and thymus of wild-type and _puma_−/− mice after combined treatment with γ-irradiation (1.5 Gy) plus dexamethasone (250 μg) on days 0 and 7. Mean ± SEM, n = 2. (B) Kaplan-Meier curves showing percentage tumor-free survival of mice after four weekly combined treatments with γ-irradiation (1.5 Gy) and dexamethasone. For wild-type mice, n = 21; for puma+/− mice, n = 22; and for _puma_−/− mice, n = 10 (P > 0.35 for all comparisons). (C) Impact of combined treatment with γ-irradiation and dexamethasone on numbers of LSK cells in wild-type and _puma_−/− mice. LSK cells were analyzed 3 d following treatment with γ-irradiation (1.5 Gy) or γ-irradiation (1.5 Gy) plus dexamethasone (250 μg). Mean ± SEM, n = 4, (**) P < 0.01; (***) P < 0.001. (D) Cell cycle status of LSK cells from mice treated as in C. Mean ± SEM, n = 3. Supplemental Figures 8 and 9 provide FACS data of the cell cycle analysis. (E) Model of thymic lymphoma development in Noxa- and Puma-deficient mice (see the text). We propose that, in wild-type mice, the γ-irradiation both creates oncogenic lesions within rare cells of the hematopoietic stem/progenitor cell compartment and, by killing most differentiated leukocytes, recruits those cells into the cell cycle to repopulate the compartment. In Noxa-deficient mice, the differentiated leukocytes die normally, but more of the stem/progenitor cells bearing potentially oncogenic mutations survive, thereby hastening tumorigenesis. In Puma-deficient mice, however, the survival of most differentiated cells removes the impetus for recruitment of the mutant stem/progenitor cells that would otherwise found a tumor.
Similar articles
- Deletion of Irf5 protects hematopoietic stem cells from DNA damage-induced apoptosis and suppresses γ-irradiation-induced thymic lymphomagenesis.
Bi X, Feng D, Korczeniewska J, Alper N, Hu G, Barnes BJ. Bi X, et al. Oncogene. 2014 Jun 19;33(25):3288-97. doi: 10.1038/onc.2013.295. Epub 2013 Aug 5. Oncogene. 2014. PMID: 23912454 - Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation.
Labi V, Erlacher M, Krumschnabel G, Manzl C, Tzankov A, Pinon J, Egle A, Villunger A. Labi V, et al. Genes Dev. 2010 Aug 1;24(15):1602-7. doi: 10.1101/gad.1940210. Genes Dev. 2010. PMID: 20679395 Free PMC article. - Combined loss of PUMA and p21 accelerates c-MYC-driven lymphoma development considerably less than loss of one allele of p53.
Valente LJ, Grabow S, Vandenberg CJ, Strasser A, Janic A. Valente LJ, et al. Oncogene. 2016 Jul 21;35(29):3866-71. doi: 10.1038/onc.2015.457. Epub 2015 Dec 7. Oncogene. 2016. PMID: 26640149 - p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa.
Valente LJ, Gray DH, Michalak EM, Pinon-Hofbauer J, Egle A, Scott CL, Janic A, Strasser A. Valente LJ, et al. Cell Rep. 2013 May 30;3(5):1339-45. doi: 10.1016/j.celrep.2013.04.012. Epub 2013 May 9. Cell Rep. 2013. PMID: 23665218 - Of the many cellular responses activated by TP53, which ones are critical for tumour suppression?
Thomas AF, Kelly GL, Strasser A. Thomas AF, et al. Cell Death Differ. 2022 May;29(5):961-971. doi: 10.1038/s41418-022-00996-z. Epub 2022 Apr 8. Cell Death Differ. 2022. PMID: 35396345 Free PMC article. Review.
Cited by
- Bid-ding for mercy: twisted killer in action.
Egle A, Asslaber D, Villunger A, Pinon-Hofbauer J. Egle A, et al. Cell Death Differ. 2013 Jul;20(7):847-9. doi: 10.1038/cdd.2013.40. Cell Death Differ. 2013. PMID: 23749178 Free PMC article. No abstract available. - A fate worse than death: apoptosis as an oncogenic process.
Ichim G, Tait SW. Ichim G, et al. Nat Rev Cancer. 2016 Aug;16(8):539-48. doi: 10.1038/nrc.2016.58. Epub 2016 Jul 1. Nat Rev Cancer. 2016. PMID: 27364482 Review. - Loss of PIDD limits NF-κB activation and cytokine production but not cell survival or transformation after DNA damage.
Bock FJ, Krumschnabel G, Manzl C, Peintner L, Tanzer MC, Hermann-Kleiter N, Baier G, Llacuna L, Yelamos J, Villunger A. Bock FJ, et al. Cell Death Differ. 2013 Apr;20(4):546-57. doi: 10.1038/cdd.2012.152. Epub 2012 Dec 14. Cell Death Differ. 2013. PMID: 23238565 Free PMC article. - The Coming Decade of Cell Death Research: Five Riddles.
Green DR. Green DR. Cell. 2019 May 16;177(5):1094-1107. doi: 10.1016/j.cell.2019.04.024. Cell. 2019. PMID: 31100266 Free PMC article. Review. - Identification of BBC3 as a novel indicator for predicting prostate cancer development and olaparib resistance.
Ma J, Qin X, Le W, Chen X, Wang X, Xu C. Ma J, et al. Discov Oncol. 2024 Sep 27;15(1):496. doi: 10.1007/s12672-024-01373-7. Discov Oncol. 2024. PMID: 39331229 Free PMC article.
References
- Allan JM, Travis LB 2005. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer 5: 943–955 - PubMed
- Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH 1993. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 362: 849–852 - PubMed
- Egle A, Harris AW, Bath ML, O'Reilly L, Cory S 2004a. VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood 103: 2276–2283 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous