Proline and hydroxyproline metabolism: implications for animal and human nutrition - PubMed (original) (raw)
Review
Proline and hydroxyproline metabolism: implications for animal and human nutrition
Guoyao Wu et al. Amino Acids. 2011 Apr.
Abstract
Proline plays important roles in protein synthesis and structure, metabolism (particularly the synthesis of arginine, polyamines, and glutamate via pyrroline-5-carboxylate), and nutrition, as well as wound healing, antioxidative reactions, and immune responses. On a per-gram basis, proline plus hydroxyproline are most abundant in collagen and milk proteins, and requirements of proline for whole-body protein synthesis are the greatest among all amino acids. Therefore, physiological needs for proline are particularly high during the life cycle. While most mammals (including humans and pigs) can synthesize proline from arginine and glutamine/glutamate, rates of endogenous synthesis are inadequate for neonates, birds, and fish. Thus, work with young pigs (a widely used animal model for studying infant nutrition) has shown that supplementing 0.0, 0.35, 0.7, 1.05, 1.4, and 2.1% proline to a proline-free chemically defined diet containing 0.48% arginine and 2% glutamate dose dependently improved daily growth rate and feed efficiency while reducing concentrations of urea in plasma. Additionally, maximal growth performance of chickens depended on at least 0.8% proline in the diet. Likewise, dietary supplementation with 0.07, 0.14, and 0.28% hydroxyproline (a metabolite of proline) to a plant protein-based diet enhanced weight gains of salmon. Based on its regulatory roles in cellular biochemistry, proline can be considered as a functional amino acid for mammalian, avian, and aquatic species. Further research is warranted to develop effective strategies of dietary supplementation with proline or hydroxyproline to benefit health, growth, and development of animals and humans.
Figures
Fig. 1
Structures of proline and related metabolites. All of these proline metabolites occur in animals, with 4-hydroxyproline being the most abundant
Fig. 2
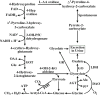
Metabolism of 4-hydroxyproline in animals. AA amino acid, AGT alanine-glyoxylate aminotransferase, Ala alanine, DAO D-amino acid oxidase, Glu glutamate, GO glycolate oxidase, GOT glutamate oxaloacetate transaminase, GR glyoxylate reductase, 4-Hyp 4-hydroxy-proline, 4-OH-2-KG 4-hydroxy-2-ketoglutarate, 3-OH-P5C Δ1-pyrro-line-3-hydroxy-5-carboxylate, LDH lactate dehydrogenase, OAA oxaloacetate, PDH pyruvate dehydrogenase, Pyr pyruvate
Fig. 3
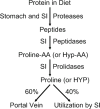
Digestion of dietary protein-bound proline or hydroxyproline (Hyp) in the gastrointestinal tract of the small intestine. Proteases and peptidases in the lumen of the small intestine hydrolyze proteins and large peptides, respectively, to eventually form proline- or hydroxyproline-containing dipeptides. These dipeptides are hydrolyzed by specific proline peptidases (prolidases) to yield free proline or hydroxyproline. Approximately 40% of luminal proline is catabolized by the mammalian small intestine, and the responsible cell types include enterocytes (Wu 1997) and bacteria (Dai et al. 2010). SI small intestine
Fig. 4
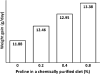
Effect of supplementing proline to a chemically purified diet on growth performance of young chickens. Data are taken from Graber et al. (1970). Chicks (the cross of New Hampshire males to Columbian females) were fed a purified diet supplemented with 0, 0.2, 0.4, and 0.8% L-proline for 6 days. The basal diet contained the following amino acids (% of diet): L-arginine–HCl, 1.21; L-histidine–HCl·H2O, 0.41; L-lysine–HCl, 1.19; L-tyrosine, 0.45; L-tryptophan, 0.15; L-phenylalanine, 0.50; DL-methionine, 0.35; L-cystine, 0.35; L-threonine, 0.65; L-leucine, 1.20; L-isoleucine, 0.60; L-valine, 0.82; glycine, 1.20; and l-glutamate, 10.00. Supplementing L-proline to the basal diet resulted in a linear increase (P < 0.01) in weight gain
Fig. 5
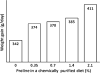
Effect of supplementing proline to a chemically purified diet on growth performance of young pigs. Data are taken from Kirchgessner et al. (1995). Young pigs were fed a purified diet supplemented with 0, 0.35, 0.7, 1.4, and 2.1% L-proline. The basal diet contained the following amino acids (% of diet): L-lysine–HCl, 1.82; DL-methionine, 0.48; L-cystine, 0.44; L-threonine, 1.06; L-tryptophan, 0.26; L-isoleucine, 0.86; L-leucine, 1.58; L-phenylalanine, 0.86; L-tyrosine, 0.86%; L-histidine, 0.56; L-valine, 1.08; L-alanine, 1.67; L-arginine, 0.48; L-aspartate, 3.61; L-glutamate, 2.02; glycine, 1.54; and L-serine, 2.24. Supplementing L-proline to the basal diet resulted in a linear increase (P < 0.01) in weight gain
Similar articles
- Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth.
Li P, Wu G. Li P, et al. Amino Acids. 2018 Jan;50(1):29-38. doi: 10.1007/s00726-017-2490-6. Epub 2017 Sep 20. Amino Acids. 2018. PMID: 28929384 Review. - The "ideal protein" concept is not ideal in animal nutrition.
Wu G, Li P. Wu G, et al. Exp Biol Med (Maywood). 2022 Jul;247(13):1191-1201. doi: 10.1177/15353702221082658. Epub 2022 Apr 11. Exp Biol Med (Maywood). 2022. PMID: 35410533 Free PMC article. - Comparative aspects of tissue glutamine and proline metabolism.
Bertolo RF, Burrin DG. Bertolo RF, et al. J Nutr. 2008 Oct;138(10):2032S-2039S. doi: 10.1093/jn/138.10.2032S. J Nutr. 2008. PMID: 18806120 Review. - Proline precursors to sustain Mammalian collagen synthesis.
Barbul A. Barbul A. J Nutr. 2008 Oct;138(10):2021S-2024S. doi: 10.1093/jn/138.10.2021S. J Nutr. 2008. PMID: 18806118 - Hydroxyproline in animal metabolism, nutrition, and cell signaling.
Hu S, He W, Wu G. Hu S, et al. Amino Acids. 2022 Apr;54(4):513-528. doi: 10.1007/s00726-021-03056-x. Epub 2021 Aug 3. Amino Acids. 2022. PMID: 34342708 Review.
Cited by
- Amino acid composition in eyes from zebrafish (Danio rerio) and sardine (Sardina pilchardus) at the larval stage.
Falco F, Barra M, Cammarata M, Cuttitta A, Jia S, Bonanno A, Mazzola S, Wu G. Falco F, et al. Springerplus. 2016 Apr 26;5:519. doi: 10.1186/s40064-016-2137-1. eCollection 2016. Springerplus. 2016. PMID: 27186483 Free PMC article. - iHyd-PseAAC (EPSV): Identifying Hydroxylation Sites in Proteins by Extracting Enhanced Position and Sequence Variant Feature via Chou's 5-Step Rule and General Pseudo Amino Acid Composition.
Ehsan A, Mahmood MK, Khan YD, Barukab OM, Khan SA, Chou KC. Ehsan A, et al. Curr Genomics. 2019 Feb;20(2):124-133. doi: 10.2174/1389202920666190325162307. Curr Genomics. 2019. PMID: 31555063 Free PMC article. - Phosphorylation of human placental aromatase CYP19A1.
Ghosh D, Egbuta C, Kanyo JE, Lam TT. Ghosh D, et al. Biochem J. 2019 Nov 15;476(21):3313-3331. doi: 10.1042/BCJ20190633. Biochem J. 2019. PMID: 31652308 Free PMC article. - Concentrate supplementation improves cold-season environmental fitness of grazing yaks: responsive changes in the rumen microbiota and metabolome.
Yi S, Wu H, Liu Y, Dai D, Meng Q, Chai S, Liu S, Zhou Z. Yi S, et al. Front Microbiol. 2023 Aug 28;14:1247251. doi: 10.3389/fmicb.2023.1247251. eCollection 2023. Front Microbiol. 2023. PMID: 37700865 Free PMC article. - 1H NMR-Based Chemometrics to Gain Insights Into the Bran of Radiation-Induced Colored Wheat Mutant.
Kil YS, Han AR, Hong MJ, Kim JB, Park PH, Choi H, Nam JW. Kil YS, et al. Front Nutr. 2022 Jan 4;8:806744. doi: 10.3389/fnut.2021.806744. eCollection 2021. Front Nutr. 2022. PMID: 35059428 Free PMC article.
References
- Aksnes A, Mundheim H, Toppe J, et al. The effect of dietary hydroxyproline supplementation on salmon (Salmo salar L.) fed high plant protein diets. Aquaculture. 2006;275:242–249.
- Austic RE. Nutritional and metabolic interrelationships of arginine, glutamic acid and proline in the chicken. Fed Proc. 1976;35:1914–1916. - PubMed
- Baker DH. Advances in protein-amino acid nutrition of poultry. Amino Acids. 2009;37:29–41. - PubMed
- Ball RO, Atkinson JL, Bayley HS. Proline as an essential amino acid for the young pig. Br J Nutr. 1986;55:659–668. - PubMed
- Barbul A. Proline precursors to sustain mammalian collagen synthesis. J Nutr. 2008;138:2021S–2024S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources