SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane - PubMed (original) (raw)
SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane
Benjamin E L Lauffer et al. J Cell Biol. 2010.
Abstract
Postsynaptic density 95/discs large/zonus occludens-1 (PDZ) domain-interacting motifs, in addition to their well-established roles in protein scaffolding at the cell surface, are proposed to act as cis-acting determinants directing the molecular sorting of transmembrane cargo from endosomes to the plasma membrane. This hypothesis requires the existence of a specific trans-acting PDZ protein that mediates the proposed sorting operation in the endosome membrane. Here, we show that sorting nexin 27 (SNX27) is required for efficient PDZ-directed recycling of the beta(2)-adrenoreceptor (beta(2)AR) from early endosomes. SNX27 mediates this sorting function when expressed at endogenous levels, and its recycling activity requires both PDZ domain-dependent recognition of the beta(2)AR cytoplasmic tail and Phox homology (PX) domain-dependent association with the endosome membrane. These results identify a discrete role of SNX27 in PDZ-directed recycling of a physiologically important signaling receptor, and extend the concept of cargo-specific molecular sorting in the recycling pathway.
Figures
Figure 1.
Efficient internalization and recycling of the β2AR in HEK293 cells simultaneously depleted of NHERFs 1 and 2. (A) Schematic of NHERF1 (also called EBP50) and NHERF2 (also called E3KARP) domain organization, showing PDZ domains and ERM protein-binding domain (EBD). (B) Verification of dual NHERF1 + 2 knockdown by immunoblotting. Arrows indicate specific bands; nonspecific bands (NS) verify equal loading between the indicated samples. Molecular mass markers (in kilodaltons) are indicated. Images shown are representative of three experiments. (C) Flow cytometric assessment of receptor internalization and recycling. HEK293 cells stably expressing either FLAG-tagged, wild-type β2AR, or the FLAG-β2AR-Ala PDZ mutant were transfected with the indicated siRNAs and assayed for surface receptor immunoreactivity before and after an agonist pretreatment and washout using fluorescence flow cytometry. (D) Recycling efficiency calculated from data shown in C, as described in Materials and methods. All error bars indicate SEM. P-values: Student’s t test with the negative control condition; n = 3 or 4.
Figure 2.
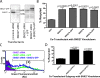
SNX27 is a distinct NHERF-related PDZ protein that interacts with the β2AR in early endosomes. (A) Schematic of SNX27 domain organization showing the PDZ domain, the PX domain, and the alternatively spliced C-terminal region that distinguishes a and b isoforms of SNX27 (a/b). Expanded box shows sequence comparison of the SNX27 PDZ domain (residues 43–133) with the first PDZ domain of NHERF1 (residues 14–91) and NHERF2 (residues 11–88). Sequences obtained from the Swiss/UniProt database were aligned with ClustalW (Thompson et al., 1994) and displayed with ESPript (Gouet et al., 1999). Secondary structure elements of the first PDZ domain of NHERF1 (Protein Data Bank accession No. 1g9o) are indicated above the alignment. Conserved residues in the three PDZ domains are shown in bold. Residues critical for recognition of peptide side chains at positions P0, P-1, P-2, and P-3 are shown in cyan, yellow, red, and blue, respectively (Appleton et al., 2006). (B) Interaction of the β2AR-derived tail sequence with the SNX27-derived PDZ domain. Purified, recombinant PDZ domain was mixed in increasing concentration with FITC-labeled peptides corresponding to the six C-terminal residues of the wild-type β2AR (a transplantable recycling sequence, solid line), or an alanine-extended version that lacks detectable recycling activity (broken line). Kd was estimated by single site fit. The plots shown are representative of three independent experiments; error bars reflect a representative SD of triplicate samples. (C) FLAG-β2AR–expressing HEK293 cells were transfected with the indicated GFP-tagged PDZ protein. After internalization of antibody-labeled receptors stimulated by 10 µM isoproterenol for 25 min, cells were fixed and imaged using dual-channel, laser-scanning confocal microscopy to reveal subcellular localization of the indicated protein. (D) FLAG-β2AR– and SNX27-GFP–transfected cells were further stained for endocytic markers that were imaged in a third fluorescent channel. All images show a middle z section and are representative of at least three independent experiments. Merged images contain boxed insets enlarged 2× from the indicated regions. Bars, 5 µm.
Figure 3.
Depletion of endogenous SNX27 prevents PDZ-directed recycling of β2ARs and accelerates down-regulation. (A) Representative immunoblot analysis (n = 4) of siRNA-mediated knockdown of the indicated PDZ protein in β2AR-expressing HEK293 cells. NHERF2 (arrow) resolves above a nonspecific band that verifies equal loading. Apparent molecular masses are indicated in parentheses. (B) Effect of knockdowns on FLAG-β2AR recycling assessed by fluorescence flow cytometry 50 min after agonist removal from the culture medium. Error bars indicate SEM. P-values: Student’s t test with negative control results; n = 4–6. (C) Time course of FLAG-β2AR recycling in cells transfected with control (solid line) compared with SNX27 siRNA (broken line). Error bars indicate SEM; n = 4. (D) Effect of SNX27 depletion on turnover of surface-biotinylated FLAG-β2AR (top) and FLAG-β2AR-Ala (bottom) after incubation of cells for the indicated time period with 10 µM isoproterenol. K, kilodaltons. (E) Quantification of the loss of biotinylated, FLAG-tagged receptors after the 5-h exposure to 10 µM isoproterenol. Error bars indicate SEM. P-values: Student’s t test for the SNX27 effect on degradation for each receptor; n = 3.
Figure 4.
Transgenic rescue of β2AR recycling by recombinant SNX27. (A) Immunoblot showing depletion of endogenous SNX27 by silencing relative to negative control siRNA (lanes 1 and 2), and replacement by cotransfection of SNX27-GFP but not GFP control plasmid (lanes 3 and 4). Electrophoretic mobilities of endogenous and SNX27 and recombinant SNX27-GFP are indicated by arrows. K, kilodaltons. (B) Flow cytometric analysis showing rescue of FLAG-β2AR recycling in SNX27 knockdown cells by cotransfection of either isoform of recombinant rat SNX27, or their GFP-tagged counterparts. (C and D) Verification of transgenic rescue using dual-channel fluorescence flow cytometry and gating of recycling data based on recombinant SNX27 expression. (C) A representative fluorescence intensity histogram of the GFP channel. The region indicated by (+) represents the populations used to verify transgenic rescue of recycling. (D) FLAG-β2AR recycling measured specifically in the SNX27/GFP (+) population. Error bars indicate SEM. P-values: Student’s t test with GFP control; n = 4–6 experiments.
Figure 5.
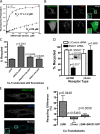
The recycling activity of SNX27 requires both its PDZ domain–mediated interaction with cargo and PX domain–mediated association with endosomes. (A) Fluorescence polarization analysis demonstrating the ability of the H112A mutation of the SNX27 PDZ domain to disrupt binding to the wild-type β2AR-derived PDZ motif. Representative saturation plots for the wild-type PDZ domain (solid line) and H112A mutant PDZ domain (broken line) are shown. (B) Representative examples of fluorescence localization patterns of PDZ mutant (H112A) and PX mutant (ΔPX) versions of SNX27, relative to FLAG-β2AR and EEA1, verifying that the PX domain is specifically required for early endosome localization of SNX27, whereas the PDZ domain is not. Bar, 5 µm. (C) Flow cytometric analysis of FLAG-β2AR recycling in SNX27 knockdown cells also transfected with a GFP, SNX27a-GFP, or SNX27a-GFP containing a mutated PDZ domain (H112A) or deleted PX domain (ΔPX). Error bars indicate SEM. P-values: Student’s t test comparison to the empty GFP control; n = 4. (D) Flow cytometric analysis showing that SNX27 depletion specifically prevents PDZ motif–directed recycling of FLAG-β2AR (first and second bars from the left; these data are replotted from Fig. 3 for comparison) without detectably affecting recycling directed by a distinct, non-PDZ sorting sequence (FLAG-β2-mrs, rightmost two bars). The inset shows a representative immunoblot confirming efficient knockdown of SNX27 in the FLAG-β2-mrs–expressing HEK293 cells used in the recycling assays (left lane, negative control; right lane, SNX27 siRNA transfection). K, kilodaltons. (E) SNX27-GFP and FLAG-β2AR were coexpressed in A10 aortic smooth muscle cells. FLAG-β2AR present in the plasma membrane was labeled and internalized as described in Materials and methods. Representative confocal images showing SNX27-GFP (top), FLAG-β2AR (middle), and a merged image (bottom). Colocalization of SNX27-GFP with β2AR-containing endosomes are enlarged 2× in the inset. Bar, 20 µm. (F) The effect of SNX27 depletion on FLAG-β2AR or FLAG-β2-mrs recycling was measured in A10 cells by antibody efflux, as described in Materials and methods. Bars represent the reduction of recycling efficiency produced by SNX27 knockdown, measured 50 min after agonist removal from the culture medium. The third bar from the right shows a rescue condition, where the relative effect of SNX27 siRNA on FLAG-β2AR recycling was assessed in the presence of recombinant SNX27. Error bars indicate SEM of recycling differences. P-values: paired t test of recycling percentage in negative control versus SNX27 siRNA-transfected conditions; n = 3–7.
Similar articles
- A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer.
Gallon M, Clairfeuille T, Steinberg F, Mas C, Ghai R, Sessions RB, Teasdale RD, Collins BM, Cullen PJ. Gallon M, et al. Proc Natl Acad Sci U S A. 2014 Sep 2;111(35):E3604-13. doi: 10.1073/pnas.1410552111. Epub 2014 Aug 18. Proc Natl Acad Sci U S A. 2014. PMID: 25136126 Free PMC article. - SNX27 regulates DRA activity and mediates its direct recycling by PDZ-interaction in early endosomes at the apical pole of Caco2 cells.
Bannert K, Berlin P, Reiner J, Lemcke H, David R, Engelmann R, Lamprecht G. Bannert K, et al. Am J Physiol Gastrointest Liver Physiol. 2020 May 1;318(5):G854-G869. doi: 10.1152/ajpgi.00374.2019. Epub 2020 Mar 2. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32116023 - Sorting Nexin 27 as a potential target in G protein‑coupled receptor recycling for cancer therapy (Review).
Bao Z, Zhou S, Zhou H. Bao Z, et al. Oncol Rep. 2020 Nov;44(5):1779-1786. doi: 10.3892/or.2020.7766. Epub 2020 Sep 14. Oncol Rep. 2020. PMID: 33000258 Free PMC article. Review. - Retromer and sorting nexins in endosomal sorting.
Gallon M, Cullen PJ. Gallon M, et al. Biochem Soc Trans. 2015 Feb;43(1):33-47. doi: 10.1042/BST20140290. Biochem Soc Trans. 2015. PMID: 25619244 Review.
Cited by
- Global Mapping of the Inc-Human Interactome Reveals that Retromer Restricts Chlamydia Infection.
Mirrashidi KM, Elwell CA, Verschueren E, Johnson JR, Frando A, Von Dollen J, Rosenberg O, Gulbahce N, Jang G, Johnson T, Jäger S, Gopalakrishnan AM, Sherry J, Dunn JD, Olive A, Penn B, Shales M, Cox JS, Starnbach MN, Derre I, Valdivia R, Krogan NJ, Engel J. Mirrashidi KM, et al. Cell Host Microbe. 2015 Jul 8;18(1):109-21. doi: 10.1016/j.chom.2015.06.004. Epub 2015 Jun 25. Cell Host Microbe. 2015. PMID: 26118995 Free PMC article. - β1-adrenergic receptor recycles via a membranous organelle, recycling endosome, by binding with sorting nexin27.
Nakagawa T, Asahi M. Nakagawa T, et al. J Membr Biol. 2013 Jul;246(7):571-9. doi: 10.1007/s00232-013-9571-6. Epub 2013 Jun 19. J Membr Biol. 2013. PMID: 23780416 Free PMC article. - PX-FERM proteins: A link between endosomal trafficking and signaling?
Ghai R, Collins BM. Ghai R, et al. Small GTPases. 2011 Sep;2(5):259-263. doi: 10.4161/sgtp.2.5.17276. Epub 2011 Sep 1. Small GTPases. 2011. PMID: 22292128 Free PMC article. - Protein interacting with C kinase 1 (PICK1) reduces reinsertion rates of interaction partners sorted to Rab11-dependent slow recycling pathway.
Madsen KL, Thorsen TS, Rahbek-Clemmensen T, Eriksen J, Gether U. Madsen KL, et al. J Biol Chem. 2012 Apr 6;287(15):12293-308. doi: 10.1074/jbc.M111.294702. Epub 2012 Feb 2. J Biol Chem. 2012. PMID: 22303009 Free PMC article. - 14-3-3 signal adaptor and scaffold proteins mediate GPCR trafficking.
Yuan L, Barbash S, Kongsamut S, Eishingdrelo A, Sakmar TP, Eishingdrelo H. Yuan L, et al. Sci Rep. 2019 Aug 1;9(1):11156. doi: 10.1038/s41598-019-47478-w. Sci Rep. 2019. PMID: 31371790 Free PMC article.
References
- Appleton B.A., Zhang Y., Wu P., Yin J.P., Hunziker W., Skelton N.J., Sidhu S.S., Wiesmann C. 2006. Comparative structural analysis of the Erbin PDZ domain and the first PDZ domain of ZO-1. Insights into determinants of PDZ domain specificity. J. Biol. Chem. 281:22312–22320 10.1074/jbc.M602901200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DA010711/DA/NIDA NIH HHS/United States
- DA012864/DA/NIDA NIH HHS/United States
- R29 DA010711/DA/NIDA NIH HHS/United States
- R01 DA012864/DA/NIDA NIH HHS/United States
- R01 DA010711/DA/NIDA NIH HHS/United States
- PN2 EY016546/EY/NEI NIH HHS/United States
- DA010711/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous