The APC/C recruits cyclin B1-Cdk1-Cks in prometaphase before D box recognition to control mitotic exit - PubMed (original) (raw)
The APC/C recruits cyclin B1-Cdk1-Cks in prometaphase before D box recognition to control mitotic exit
Wouter van Zon et al. J Cell Biol. 2010.
Abstract
The ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C) is activated at prometaphase by mitotic phosphorylation and binding of its activator, Cdc20. This initiates cyclin A degradation, whereas cyclin B1 is stabilized by the spindle checkpoint. Upon checkpoint release, the RXXL destruction box (D box) was proposed to direct cyclin B1 to core APC/C or Cdc20. In this study, we report that endogenous cyclin B1-Cdk1 is recruited to checkpoint-inhibited, phosphorylated APC/C in prometaphase independently of Cdc20 or the cyclin B1 D box. Like cyclin A, cyclin B1 binds the APC/C by the Cdk cofactor Cks and the APC3 subunit. Prior binding to APC/C(Cdc20) makes cyclin B1 a better APC/C substrate in metaphase, driving mitotic exit and cytokinesis. We conclude that in prometaphase, the phosphorylated APC/C can recruit both cyclin A and cyclin B1 in a Cks-dependent manner. This suggests that the spindle checkpoint blocks D box recognition of APC/C-bound cyclin B1, whereas distinctive complexes between the N terminus of cyclin A and Cdc20 evade checkpoint control.
Figures
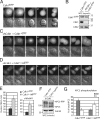
Figure 1.
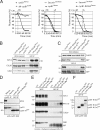
Cdk1-bound Cks proteins enhance APC/C phosphorylation and mitotic progression. (A) Cdk1–EYFP shows no distinct intracellular localization. M denotes metaphase alignment and onset of cyclin B1 destruction in U2OS cells. (B) Complementation of Cdk1 RNAi by RNAi-resistant Cdk1*–EYFP. (C) Reconstitution of Cdk1 depletion by Cdk1*–EYFP reveals cyclin B1 binding (
Fig. S1 B
and
Video 1
). Cdk1 localization gets dispersed upon cyclin B1 degradation at metaphase. (D) Complementation of Cdk1 depletion by non-Cks–binding Cdk1*–1N delays cells in mitosis. (E) Reconstituted expression of fluorescent wild-type Cdk1 rescues Cdk1 RNAi (Lindqvist et al., 2007); NEB to anaphase (NEB-A) is normal at ∼24 min (n = 8). Cdk1*–1N–EYFP-complemented cells are delayed in mitosis, NEB to anaphase is 57 min (n = 7). (right) Graph shows that Cdk1*–1N–EYFP-complemented cells delay independently of the spindle checkpoint. Knockdown of BubR1 (shBubR1; Lens et al., 2003) was verified by staining imaged cells with anti-BubR1 after fixation (not depicted). (F) Mitotic cells arrested in the spindle checkpoint were collected. Extracts were blotted with the indicated antibodies. (G) Intensities of the most phosphorylated forms of APC3 (ppp) and the unphosphorylated APC3 (u) from four different experiments as in F were quantified. A detailed description is shown in Fig. S1 E. WCE, whole cell extract. Error bars indicate standard deviations. Markers are given in kilodaltons. Bars: (A) 5 µM; (C and D) 10 µM.
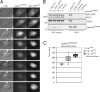
Figure 2.
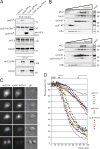
Cks promotes APC/C–dependent destruction of cyclin B1 and securin. (A and B) Cells were treated with siRNA pools targeting Cks1 (siCks1; control) or both Cks1 and Cks2 (siCks1 and 2). Total amounts of siRNA were kept equal. Cells transfected with either securin-Venus (A) or cyclin B1–Venus (B) and expressing similar fluorescent protein levels were followed during mitosis (see
Fig. S2 A
for quantified fluorescence levels and total destruction times). (C) Fluorescence levels plotted over time starting 3 min before NEB are shown. Data are representative of three independent experiments for each condition. Numbers indicate minutes. Bars, 10 µM.
Figure 3.
Cks proteins direct cyclin B1 binding to the phosphorylated APC/C. (A) Cells were treated with siRNA pools targeting Cks1 (siCks1; control, or both Cks1 and Cks2; siCks1 + 2). The cells were thymidine released, blocked in nocodazole, and collected by mitotic shake off. Extracts were blotted with the indicated antibodies. IPs with anti–cyclin B1 antibodies were performed and blotted to examine coprecipitation of the indicated proteins. APC3 binding in cyclin B1 IPs correlates with the hyper-phosphorylated APC3 (APC3-PPP) present in extracts. (B) Cells were treated with siRNA pools targeting Plk1 (siPlk1), both Cks1 and Cks2 (siCks), or Plk1, Cks1, and Cks2 (siPlk1 + siCks). Mitotic cells were collected as in A, and extracts were Western blotted and probed with anti-Cks1 antibody. (top) A background band detected by the Cks antiserum (asterisk) is shown. Serial dilutions of control (ctrl) mitotic cells show that Cks1 knockdown is ∼75% after siCks or between 50 and 75% after siPlk1 + siCks. (C) The same lysates shown in B were used for IP of cyclin B1 and blotted to examine APC/C and Cdc20 binding. Serial dilutions of control cyclin B1 IPs are shown as a reference. Markers are given in kilodaltons. WCE, whole cell extract.
Figure 4.
Cks promotes recruitment of cyclin B1 as an APC/C substrate. (A) Cells were cotransfected with cyclin B1–cerulean and either Cdk1*–EYFP or Cdk1*–1N–EYFP. A cell undergoing mitotic exit without cytokinesis is shown. DIC, differential interference contrast. Bar, 5 µM. (B) Reduced APC/CCdc20 binding of cyclin B1–Cdk1–1N complexes in cells, whereas total APC/C phosphorylation is normal. Checkpoint-arrested mitotic cells selected for expression of indicated constructs were collected. GFP IPs (pulling down Cdk1–EYFP) on equalized extracts were blotted for coprecipitation of the indicated proteins. Cyclin B1–Cherry is not recognized by the anti-GFP antibodies. WCE, whole cell extract. (C) Quantitative comparison of destruction times of cyclin B1 bound to Cdk1 (white) or Cdk1–1N (light gray). The destruction times of the cells expressing Cdk1–1N, which failed cytokinesis, are presented in the dark gray box. The plots contain data from three independent experiments (see
Fig. S2 B
for destruction curve). Markers are given in kilodaltons.
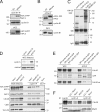
Figure 5.
Binding of phosphorylated APC/CCdc20 to cyclin B1 starts in mitosis. (A) Cyclin B1 IPs from synchronized G2 phase and nocodazole-arrested mitotic cells (mitosis) are shown. See Materials and methods for synchronization details. IPs were analyzed for coprecipitating proteins by Western blotting probed for anti-APC3 (top; showing only phosphorylated APC3) or anti–cyclin B1 (middle). (bottom) The presence of unphosphorylated (lane 1) and phosphorylated (lane 2) APC3 in whole cell extracts (WCE) used for the IPs (10% of input) is shown. (B) APC/C IPs using anti-APC6 antibodies on extracts of G2 phase cells and mitotic cells. (C) Cyclin B1 IPs on extracts from G2 or mitotic cells were used in in vitro ubiquitination assays. Mitotic cells arrested in nocodazole were released but kept in mitosis by the proteasome inhibitor MG132 (NR, MG132). Control, GFP IPs. Purified E1 enzyme, the APC/C-specific E2 enzyme UbcH10, ATP, and HA-ubiquitin (HA-Ub) were added to start the reactions. HA-ubiquitin–conjugated reaction products were probed with anti-HA antibody (for further controls see
Fig. S3
). Asterisks indicate an aspecific background band in the control GFP IPs. (D) Comparison of mitotic cells arrested in the spindle checkpoint (Noco), released from the spindle checkpoint arrest but kept in mitosis by MG132, or allowed to enter mitosis in the presence of MG132; the latter contain high cyclin A levels (top). Cyclin B1 IPs are shown; negative control, securin (Sec) IP. Western blots were analyzed for coprecipitating proteins with the indicated antibodies. (top) Phosphorylated forms of APC3 in cyclin B1 IPs are shown. (E) Cells were transfected and selected for expression of the indicated cyclin–GFP fusion proteins. Mitotic cells that had entered mitosis in the presence of the proteasome inhibitor MG132 were collected by shake off. GFP IPs were probed for coprecipitation of Cdc20. (F) Cells were synchronized in G2 and mitosis as indicated. Anti–cyclin A IPs, anti–cyclin B1 IPs, and mitotic extracts were blotted for APC3, Cdc20, and Mad2. Comparable polyclonal antibodies were used detecting the N-terminal 430 amino acids of the respective cyclins. Markers are given in kilodaltons.
Figure 6.
Retention and destruction of cyclin B1 critically depends on APC3. (A) Cells were arrested in mitosis by nocodazole after treatment with control siRNA (lanes 1 and 2) or arrested by treatment of siRNA pools targeting APC3 (lanes 3 and 4) or APC2 (lanes 5 and 6). 50% of these mitotic cells were released out of mitosis by the Cdk1 inhibitor roscovitine (Rosco; lanes 2, 4, and 6). Extracts were blotted and probed with antibodies against the indicated proteins. (B) IPs with anti–cyclin B1 antibodies on extracts of mitotic cells arrested in the spindle checkpoint (nocodazole) by allowing the cells to enter mitosis in the presence of MG132 or by treatment of siRNA pools targeting APC3 or APC2. Extracts and IPs were probed with antibodies against the indicated proteins. (C) IPs with cyclin B1 antibodies on extracts of mitotic cells arrested by the spindle checkpoint and treated with control siRNA or an siRNA pool targeting Cdc20. IPs were blotted and probed with antibodies against the indicated proteins. Identical results were obtained by using shRNA to target APC3 (
Fig. S5
). Markers are given in kilodaltons. WCE, whole cell extract.
Figure 7.
APC/CCdc20 binding to cyclin B1 correlates with a substrate retention step, preceding a D box–dependent ubiquitination step. (A) Cells were cotransfected with vectors coding for cyclin B1–Venus and ΔN–cyclin B1–ECFP (left), securin-cerulean and cyclin B1–Venus (middle), or securin-cerulean and cyclin B1–R202A–Venus (right). Cells expressing both proteins were followed through mitosis, and fluorescence levels were plotted. Metaphase (M) and anaphase (A) initiation judged from differential interference contrast images are shown. Graphs are representative of at least two independent experiments. (middle and right) The cells analyzed expressed cyclin B1 or securin at low levels, allowing onset of their destruction at chromosome alignment. With wild-type constructs, sister chromatid separation and cytokinesis were normal. The other fluorescent constructs were expressed to similar levels. At least 20 cells expressing single fusion proteins were analyzed with similar results. (B and C) Wild-type and nondegradable mutants of cyclin B1 bind equally well to APC/CCdc20 in mitosis, whereas non-Cdk–binding mutants of cyclin B1 do not bind. (B) Cells expressing Cdk-binding mutant cyclin B1–R202A–Venus (lane 1), nondegradable ΔN–cyclin B1–ECFP (lane 2), or control cyclin B1–Venus (lane 3) were synchronized in the spindle checkpoint and collected by mitotic shake off. Successful expression of the constructs was validated by fluorescence microscopy (not depicted). (C) Cells expressing control cyclin B1–Venus (lane 1), Cdk-binding mutant cyclin B1–R202A–Venus (lane 2), the non-Cdk–binding N-terminal destruction region of cyclin B1 fused to GFP (lane 3), or nondegradable R42A D box mutants (lanes 4 and 5) were synchronized in the spindle checkpoint and collected by mitotic shake off. (B and C) Fusion proteins were immunoprecipitated by anti-GFP antibodies, equally recognizing GFP or spectral variants. IPs were blotted and probed with antibodies against the indicated proteins. (D) Cells were treated with nocodazole, and cyclin B1 IPs were performed on extracts of the collected mitotic cells (lane 1) or on extracts from an asynchronous population (lane 2). Cells expressing the first 97 amino acids of cyclin B1 fused to GFP either containing an intact D box (lane 3) or an R42A-mutated D box (lane 4) were collected in mitosis. Anti-GFP antibodies were used for IP of the fusion proteins from extracts (lanes 3 and 4). IPs were probed for cyclin B1 and APC3. (lane 1) Binding of APC/C to endogenous cyclin B1 in mitosis as reference is shown. In prometaphase, binding is not detectable to the N-terminal fragments of cyclin B1. (E and F) Mitotic cells were collected by shake off after taxol treatment and released from the spindle checkpoint by addition of aurora B inhibitor ZM447439 but kept in mitosis by the addition of proteasome inhibitor MG132. After 1 h, cells were lysed and extracts used for IP of fusion proteins of GFP and the cyclin B1 N terminus (1–86), (cycB1-N (1X)-GFP), or tandem or sixfold repeats of the same region (cycB1-N (2X)-GFP and cycB1-N (6X)-GFP, respectively). IPs were blotted for APC3, Cdc20, or Cdk1 (as a negative control [ctrl]; E) or the cyclin B1 fragments (F). APC3 long exposure (APC3 le) is an overexposed blot to reveal minor APC/C binding to the single- or tandem-repeat cyclin B1 fragment. Markers are given in kilodaltons. WCE, whole cell extract.
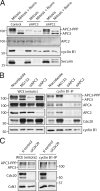
Figure 8.
Cks enhances destruction of the N-terminal cyclin B1 fragment. (A) Cells expressing cyclin B1–Venus (lane 2), the first 86 amino acids of cyclin B1 fused to Venus (cycB1-N-Venus; lane 3), or the same N-terminal cyclin B1 fragment fused to Cks1 and Venus (cycB1-N-Cks1-Venus; lane 4) was synchronized in the spindle checkpoint and mitotic cells collected by mitotic shake off. GFP IPs on equal amounts of protein precipitated the Venus fusion proteins, which were blotted to examine coprecipitation of the indicated proteins (bottom). (B) Spindle checkpoint–arrested mitotic cells expressing cyclin B1–N–Venus or cyclin B1–N–Cks–Venus were collected, and lysates were subjected to sucrose gradient centrifugation. Fractions were blotted and probed with antibodies against the indicated proteins. (C) U2OS cells were cotransfected with vectors encoding cyclin B1–N–cerulean (cycB1-N) and cyclin B1–N–Cks–Venus (cycB1-N-Cks1) and followed as they progressed through mitosis. DIC, differential interference contrast. Bar, 10 µM. (D) Venus- and cerulean-tagged versions of cyclin B1–N or cyclin B1–N–Cks1 were cotransfected in U2OS cells. Cells either expressing cyclin B1–N or cyclin B1–N–Cks1 were followed during mitosis, and fluorescence levels were measured at NEB and plotted over time. For comparison, cells were also simultaneously transfected with three differently colored constructs, cyclin B1–N–Venus, cyclin B1–N–Cks1–cerulean, and a full-length red fluorescent cyclin B1 construct, cyclin B1–Cherry. Fluorescence levels of two triple-colored cells passing mitosis and performing a normal anaphase were included in the graph as a reference. Results of two independent experiments are shown. M, metaphase onset; A, anaphase onset. Comparable differences in destruction of the cyclin B1 reporters were found in >30 cells in four independent experiments. We found no significant effect of the different fluorescent tags on the destruction rates of the cyclin B1 fragments (
Fig. S2 C
). Markers are given in kilodaltons. WCE, whole cell extract.
Similar articles
- How cyclin A destruction escapes the spindle assembly checkpoint.
Di Fiore B, Pines J. Di Fiore B, et al. J Cell Biol. 2010 Aug 23;190(4):501-9. doi: 10.1083/jcb.201001083. J Cell Biol. 2010. PMID: 20733051 Free PMC article. - Cyclin A and Nek2A: APC/C-Cdc20 substrates invisible to the mitotic spindle checkpoint.
van Zon W, Wolthuis RM. van Zon W, et al. Biochem Soc Trans. 2010 Feb;38(Pt 1):72-7. doi: 10.1042/BST0380072. Biochem Soc Trans. 2010. PMID: 20074038 Review. - How APC/C-Cdc20 changes its substrate specificity in mitosis.
Izawa D, Pines J. Izawa D, et al. Nat Cell Biol. 2011 Mar;13(3):223-33. doi: 10.1038/ncb2165. Epub 2011 Feb 20. Nat Cell Biol. 2011. PMID: 21336306 Free PMC article. - Phosphorylation of Mcl-1 by CDK1-cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest.
Harley ME, Allan LA, Sanderson HS, Clarke PR. Harley ME, et al. EMBO J. 2010 Jul 21;29(14):2407-20. doi: 10.1038/emboj.2010.112. Epub 2010 Jun 4. EMBO J. 2010. PMID: 20526282 Free PMC article. - To cell cycle, swing the APC/C.
van Leuken R, Clijsters L, Wolthuis R. van Leuken R, et al. Biochim Biophys Acta. 2008 Sep;1786(1):49-59. doi: 10.1016/j.bbcan.2008.05.002. Epub 2008 May 21. Biochim Biophys Acta. 2008. PMID: 18544349 Review.
Cited by
- Identification of SNF2h, a chromatin-remodeling factor, as a novel binding protein of Vpr of human immunodeficiency virus type 1.
Taneichi D, Iijima K, Doi A, Koyama T, Minemoto Y, Tokunaga K, Shimura M, Kano S, Ishizaka Y. Taneichi D, et al. J Neuroimmune Pharmacol. 2011 Jun;6(2):177-87. doi: 10.1007/s11481-011-9276-5. Epub 2011 Apr 26. J Neuroimmune Pharmacol. 2011. PMID: 21519849 - Cyclin B3 is a mitotic cyclin that promotes the metaphase-anaphase transition.
Yuan K, O'Farrell PH. Yuan K, et al. Curr Biol. 2015 Mar 16;25(6):811-816. doi: 10.1016/j.cub.2015.01.053. Epub 2015 Mar 5. Curr Biol. 2015. PMID: 25754637 Free PMC article. - The spindle checkpoint, APC/C(Cdc20), and APC/C(Cdh1) play distinct roles in connecting mitosis to S phase.
Clijsters L, Ogink J, Wolthuis R. Clijsters L, et al. J Cell Biol. 2013 Jun 24;201(7):1013-26. doi: 10.1083/jcb.201211019. Epub 2013 Jun 17. J Cell Biol. 2013. PMID: 23775192 Free PMC article. - Proposed megakaryocytic regulon of p53: the genes engaged to control cell cycle and apoptosis during megakaryocytic differentiation.
Apostolidis PA, Lindsey S, Miller WM, Papoutsakis ET. Apostolidis PA, et al. Physiol Genomics. 2012 Jun 15;44(12):638-50. doi: 10.1152/physiolgenomics.00028.2012. Epub 2012 May 1. Physiol Genomics. 2012. PMID: 22548738 Free PMC article. - Regulated inactivation of the spindle assembly checkpoint without functional mitotic spindles.
De Souza CP, Hashmi SB, Yang X, Osmani SA. De Souza CP, et al. EMBO J. 2011 Jun 3;30(13):2648-61. doi: 10.1038/emboj.2011.176. EMBO J. 2011. PMID: 21642954 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous