Autophagy Blockade Sensitizes Prostate Cancer Cells towards Src Family Kinase Inhibitors - PubMed (original) (raw)
doi: 10.1177/1947601909358324.
Pei-Ching Chang, Joy C Yang, Cheng-Ying Chu, Ling-Yu Wang, Nien-Tsu Chen, Ai-Hong Ma, Sonal J Desai, Su Hao Lo, Christopher P Evans, Kit S Lam, Hsing-Jien Kung
Affiliations
- PMID: 20811583
- PMCID: PMC2930266
- DOI: 10.1177/1947601909358324
Autophagy Blockade Sensitizes Prostate Cancer Cells towards Src Family Kinase Inhibitors
Zhaoju Wu et al. Genes Cancer. 2010 Jan.
Abstract
There is overwhelming evidence that tyrosine kinases play an important role in cancer development. As a prototype of targeted therapy, tyrosine kinase inhibitors are now successfully applied to cancer treatment. However, as single agents, tyrosine kinase inhibitors have not achieved satisfactory results in the treatment of prostate cancer, principally due to their inability to efficiently kill tumor cells. The authors' laboratory has been interested in the role of the Src complex in prostate cancer progression, including the induction of androgen independence and metastasis. Previously, the authors reported that Src inhibitors such as saracatinib and PP2 caused G1 growth arrest and diminished invasiveness in prostate cancer cells but rarely apoptosis. Here, they have shown that Src family kinase (SFK) inhibitors can induce a high level of autophagy, which protects treated cells from undergoing apoptosis. Src siRNA knockdown experiments confirmed that autophagy was indeed caused by the lack of Src activity. The SFK inhibitor-induced autophagy is accompanied by the inhibition of the PI3K (type I)/Akt/mTOR signaling pathway. To test whether autophagy blockade could lead to enhanced cell death, pharmacological inhibitors (3-methyladenine and chloroquine) and a genetic inhibitor (siRNA targeting Atg7) were used in combination with SFK inhibitors. The results showed that autophagy inhibition effectively enhanced cell killing induced by SFK inhibitors. Importantly, the authors showed that a combination of saracatinib with chloroquine in mice significantly reduced prostate cancer (PC3) xenograft growth compared with the control group. Taken together, these data suggest that (1) autophagy serves a protective role in SFK inhibitor-mediated cell killing, and (2) clinically acceptable autophagy modulators may be used beneficially as adjunctive therapeutic agents for SFK inhibitors.
Keywords: Src tyrosine kinase; autophagy; chloroquine; prostate cancer; saracatinib.
Conflict of interest statement
Dr Christopher P. Evans serves as a consultant to AstraZeneca, which provided saracatinib for this work. The other authors disclosed no potential conflict of interest.
Figures
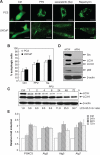
Figure 1.
Src inhibitors, PP2 and saracatinib, induce autophagy in prostate cancer cell lines LNCaP and PC3. (A) Representative micrographs of PC3-eGFP-LC3 and LNCaP-eGFP-LC3 cells showing GFP-LC3 localization. PC3-eGFP-LC3 (upper panel) and LNCaP-eGFP-LC3 (lower panel) stable cell lines expressing eGFP-LC3 were treated with DMSO (vehicle control), 10 μM PP2, or 1 μM saracatinib for 48 h and were then analyzed by fluorescence microscopy. Puncta represent autophagosome formation. PC3-eGFP-LC3 and LNCaP-eGFP-LC3 cells treated with 2 μM rapamycin for 4 h were used as a positive control. Scale bar = 30 μm. (B) Quantification plot of autophagic cell numbers in (A). The percentage of autophagic cells was determined by randomly counting 200 cells under the fluorescent microscope. Data represented as mean ± SE. (C) A time-dependent increase in the ratio of LC3-II/LC3-I under the treatment of 10 μM PP2. PC3 cells were treated with 10 μM PP2, and cell lysates were harvested at different time points as indicated and analyzed by Western blotting for LC3-I and LC3-II. β-actin was used as the loading control. (D) PC3 cells were collected at 72 h after being transfected with the negative control siRNA or 100 pmol of Src siRNA oligonucleotides. Cell lysates were then subjected to immunoblot analysis with the antibodies indicated. β-actin was detected as loading control. (E) Induction of PI3KC3, Atg3, Atg5, and Atg7 expression in PC3 cells. PI3KC3, Atg3, Atg5, and Atg7 mRNA levels were measured by quantitative real-time PCR in cells treated for 24, 48, and 72 h with 10 μM PP2. The expression levels were compared to the vehicle control DMSO-treated expression levels. Values represent mean ± SE.
Figure 2.
PP2 and saracatinib inhibit mTOR signaling pathway through the PI3K (type I)/Akt pathway. (A) Src phosphorylation in PC3 cells was inhibited in a dose-dependent manner by PP2 and saracatinib following a 30-min treatment. Cell lysates were analyzed by immunoblotting with antibodies indicated. β-actin was detected as loading control. (B) PC3 cells were treated with 10 μM PP2 (left panel)/1 μM saracatinib (right panel) for 0.5, 1, 2, 4, 8, and 24 h. Cell lysates were analyzed by immunoblotting with antibodies as indicated. Controls were treated with vehicle alone. β-actin was detected as loading control. (C) A model depicting Src-mediated signal pathway, involved in the suppression of autophagy.
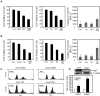
Figure 3.
Inhibition of autophagy enhances PP2 and saracatinib-induced PC3 cell death. (A, B, left and middle panels) PC3 cells were seeded in 96-well plates and cultured overnight. The following day, the cells were treated with 0.1% DMSO as vehicle control, 10 μM CQ or 1 mM 3-MA, 5 μM PP2 or 1 μM saracatinib alone or in combination with 10 μM CQ, or 1 mM 3-MA. After 48 h, cell viability was assessed by MTT assay. Significant difference between PP2/Sa and PP2/Sa plus 3-MA/CQ (P < 0.0005). Values represent mean ± SE. (A, B, right panel) PC3 cells were treated with DMSO (vehicle control), 10 μM CQ, 5 μM PP2, or 1 μM saracatinib alone or in combination with 10 μM CQ. After 24 h, the cultured cells were assayed for caspase-3/7 activity. Significant difference between PP2/Sa and PP2/Sa plus CQ (P < 0.05). Values represent mean ± SE. (C) PC3 cell death was assessed by propidium iodide (PI) staining and flow cytometry analysis after treatment with DMSO (vehicle control), 5 μM PP2, Atg7 siRNA, or 5 μM PP2 plus Atg7 siRNA for 72 h. Fluorescence-activated cell sorting (FACS) analysis shows sub-G1 content. (D, upper panel) Immunoblot for cell lysates from PC3 cells transiently transfected with negative control siRNA or Atg7 siRNA indicating the knockdown of Atg7 in PC3 cells. (D, lower panel) The percentage of sub-G1 content for (3C).
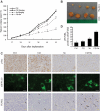
Figure 4.
Combination treatment of CQ and saracatinib synergistically inhibits tumor growth in vivo. (A) Mice with PC3-eGFP-LC3 implants were treated with vehicle, 50 mg/kg CQ, 25 mg/kg saracatinib, or CQ plus saracatinib daily. Mice were euthanized after 53 days. Tumor volumes are reported as mean ± SE. (B) Representative tumor samples, including combined treatment of 50 mg/kg CQ with 25 mg/kg saracatinib (upper panel) and vehicle control group (lower panel). (C) Representative micrographs of immunohistochemistry using anti-pSrc antibody (upper panel). Scale bar = 50 μm. Representative fluorescent microscope images of frozen tumor tissue sections for eGFP-LC3 (middle panel). Scale bar = 30 μm. Representative TdT-mediated dUTP nick end labeling (TUNEL) staining of tumor tissues (lower panel). TUNEL-positive cells are indicated by arrows. Scale bar = 50 μm. (D) Tumor sections were analyzed for percentage of apoptotic cells by TUNEL assay. Average numbers of TUNEL-positive cells were counted in 4 randomly selected fields in 3 tumor samples from each group; values represent mean ± SE.
Similar articles
- Suppression of autophagy sensitizes multidrug resistant cells towards Src tyrosine kinase specific inhibitor PP2.
Ahn JH, Lee M. Ahn JH, et al. Cancer Lett. 2011 Nov 28;310(2):188-97. doi: 10.1016/j.canlet.2011.06.034. Epub 2011 Jul 3. Cancer Lett. 2011. PMID: 21775053 - Targeting tyrosine kinases and autophagy in prostate cancer.
Kung HJ. Kung HJ. Horm Cancer. 2011 Feb;2(1):38-46. doi: 10.1007/s12672-010-0053-3. Epub 2010 Dec 2. Horm Cancer. 2011. PMID: 21350583 Free PMC article. Review. - Blocked autophagy using lysosomotropic agents sensitizes resistant prostate tumor cells to the novel Akt inhibitor AZD5363.
Lamoureux F, Thomas C, Crafter C, Kumano M, Zhang F, Davies BR, Gleave ME, Zoubeidi A. Lamoureux F, et al. Clin Cancer Res. 2013 Feb 15;19(4):833-44. doi: 10.1158/1078-0432.CCR-12-3114. Epub 2012 Dec 20. Clin Cancer Res. 2013. PMID: 23258740 - Effect of the specific Src family kinase inhibitor saracatinib on osteolytic lesions using the PC-3 bone model.
Yang JC, Bai L, Yap S, Gao AC, Kung HJ, Evans CP. Yang JC, et al. Mol Cancer Ther. 2010 Jun;9(6):1629-37. doi: 10.1158/1535-7163.MCT-09-1058. Epub 2010 May 18. Mol Cancer Ther. 2010. PMID: 20484016 - The role of Src in prostate cancer.
Fizazi K. Fizazi K. Ann Oncol. 2007 Nov;18(11):1765-73. doi: 10.1093/annonc/mdm086. Epub 2007 Apr 10. Ann Oncol. 2007. PMID: 17426060 Review.
Cited by
- Autophagy control by the VEGF-C/NRP-2 axis in cancer and its implication for treatment resistance.
Stanton MJ, Dutta S, Zhang H, Polavaram NS, Leontovich AA, Hönscheid P, Sinicrope FA, Tindall DJ, Muders MH, Datta K. Stanton MJ, et al. Cancer Res. 2013 Jan 1;73(1):160-71. doi: 10.1158/0008-5472.CAN-11-3635. Epub 2012 Nov 13. Cancer Res. 2013. PMID: 23149913 Free PMC article. - Autophagy inhibition synergistically enhances anticancer efficacy of RAMBA, VN/12-1 in SKBR-3 cells, and tumor xenografts.
Godbole AM, Purushottamachar P, Martin MS, Daskalakis C, Njar VC. Godbole AM, et al. Mol Cancer Ther. 2012 Apr;11(4):898-908. doi: 10.1158/1535-7163.MCT-11-0860. Epub 2012 Feb 14. Mol Cancer Ther. 2012. PMID: 22334589 Free PMC article. - FDA-Approved Kinase Inhibitors in Preclinical and Clinical Trials for Neurological Disorders.
Lui A, Vanleuven J, Perekopskiy D, Liu D, Xu D, Alzayat O, Elgokhy T, Do T, Gann M, Martin R, Liu DZ. Lui A, et al. Pharmaceuticals (Basel). 2022 Dec 13;15(12):1546. doi: 10.3390/ph15121546. Pharmaceuticals (Basel). 2022. PMID: 36558997 Free PMC article. Review. - Glucocorticoids downregulate Fyn and inhibit IP(3)-mediated calcium signaling to promote autophagy in T lymphocytes.
Harr MW, McColl KS, Zhong F, Molitoris JK, Distelhorst CW. Harr MW, et al. Autophagy. 2010 Oct;6(7):912-21. doi: 10.4161/auto.6.7.13290. Epub 2010 Oct 2. Autophagy. 2010. PMID: 20814235 Free PMC article. - Autophagy as a therapeutic target in cancer.
Chen N, Karantza V. Chen N, et al. Cancer Biol Ther. 2011 Jan 15;11(2):157-68. doi: 10.4161/cbt.11.2.14622. Epub 2011 Jan 15. Cancer Biol Ther. 2011. PMID: 21228626 Free PMC article. Review.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49 - PubMed
- Prezioso D, Galasso R, Di Martino M, Iapicca G, Annunziata E, Iacono F. Actual chemotherapeutical possibilities in hormone-refractory prostate cancer (HRPC) patients. Anticancer Res 2007;27:1095-104 - PubMed
- Johnstone PA, Assikis V, Goodman M, Ward KC, Riffenburgh RH, Master V. Lack of survival benefit of post-operative radiation therapy in prostate cancer patients with positive lymph nodes. Prostate Cancer Prostatic Dis 2007;10:185-88 - PubMed
- Golas JM, Lucas J, Etienne C, Golas J, Discafani C, Sridharan L, et al. SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res 2005;65:5358-64 - PubMed
- Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat 2007;105:319-26 - PubMed
Grants and funding
- R01 DK052659-12/DK/NIDDK NIH HHS/United States
- R01 DK052659/DK/NIDDK NIH HHS/United States
- R01 DK078243-04/DK/NIDDK NIH HHS/United States
- R01 CA114575-05/CA/NCI NIH HHS/United States
- R01 DK078243/DK/NIDDK NIH HHS/United States
- R01 CA114575/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous