CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content - PubMed (original) (raw)
CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content
Branch Craige et al. J Cell Biol. 2010.
Abstract
Mutations in human CEP290 cause cilia-related disorders that range in severity from isolated blindness to perinatal lethality. Here, we describe a Chlamydomonas reinhardtii mutant in which most of the CEP290 gene is deleted. Immunoelectron microscopy indicated that CEP290 is located in the flagellar transition zone in close association with the prominent microtubule-membrane links there. Ultrastructural analysis revealed defects in these microtubule-membrane connectors, resulting in loss of attachment of the flagellar membrane to the transition zone microtubules. Biochemical analysis of isolated flagella revealed that the mutant flagella have abnormal protein content, including abnormal levels of intraflagellar transport proteins and proteins associated with ciliopathies. Experiments with dikaryons showed that CEP290 at the transition zone is dynamic and undergoes rapid turnover. The results indicate that CEP290 is required to form microtubule-membrane linkers that tether the flagellar membrane to the transition zone microtubules, and is essential for controlling flagellar protein composition.
Figures
Figure 1.
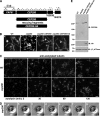
Identification of a C. reinhardtii CEP290 mutant. (A) Schematic of the region of C. reinhardtii chromosome 3 containing the CEP290 (POC3) locus. CEP290 is flanked by the FAP61 gene and by gene models encoding predicted proteins nos.182839 and 144014 in Joint Genome Institute version 4.0 of the Chlamydomonas genome (
http://genome.jgi-psf.org/Chlre4/Chlre4.home.html
). Arrows indicate the positions of PCR primer pairs; plus and minus symbols indicate whether a PCR product was generated using genomic DNA of strain Y168 as template (all primer pairs amplified a product when wild-type genomic DNA was used as template). Rescuing constructs consisted of either untagged or HA-tagged genomic DNA fragments containing only the CEP290 gene. (B) 30-frame image averages of videos (acquired at 30 frames per second) depicting the mutant phenotype (cep290) and rescue of the phenotype (cep290::CEP290 and cep290::CEP290-HA). Motile cells are seen as long meandering tracks, whereas nonmotile (palmelloid) cells appear as bright foci (cep290). See
Videos 1–4
. Bar, 10 µm. (C) Palmelloid cep290 mutant cells were released from the mother cell wall by treatment with autolysin, then fixed at the indicated time points after the addition of autolysin and processed for immunofluorescence using antibodies to acetylated tubulin. The mutant cells have stumpy flagella that partially elongate over time. Bar, 10 µm. (D) cep290 mutant cells were hatched with autolysin, immobilized in 0.5% agar, and imaged at the indicated time points (minutes) after the addition of autolysin. The arrowhead marks a flagellum that began to elongate then formed a bulge. The arrow marks a flagellum that failed to elongate and formed a bulge. Bar, 1 µm. (E) Western blot of whole cells. A peptide antibody generated against the C-terminal 14 amino acids of C. reinhardtii CEP290 is specific and confirms the absence of CEP290 in the mutant strain and expression of CEP290 in the rescued strains. βF1-ATPase is a mitochondrial protein used as a loading control. The positions of standard proteins and their molecular masses in kD are indicated.
Figure 2.
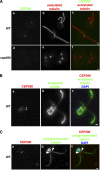
C. reinhardtii CEP290 is located in the transition zone. (A) Wild-type and cep290 mutant cells were fixed and processed for immunofluorescence using the indicated antibodies. (B and C) Wild-type cells were detergent-extracted, and the resulting cytoskeletons were processed for immunofluorescence using the indicated antibodies. Co-labeling with antibodies to CEP290 and acetylated tubulin revealed CEP290 localization at the base of each flagellum (A and B). (C) Co-labeling with polyglutamylated tubulin indicated that CEP290 is located within the transition zone (arrowheads; Lechtreck and Geimer, 2000). Also see
Fig. S1
. Bars, 1 µm.
Figure 3.
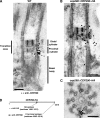
Loss of CEP290 causes defects in the microtubule–membrane connections within the transition zone. (A and B) Longitudinal sections through the transition zone (brackets) reveal that the electron-dense wedge-shaped structures (arrowheads in A) between the transition zone microtubules and flagellar membrane are missing in the cep290 mutant. (C and D) Cross sections reveal that the Y-shaped connectors (arrowheads in C) that bridge the transition zone microtubules with the membrane are missing from most cep290 doublet microtubules. Insets in C and D are image averages of 30 transition zone doublet microtubules. (E–G) Some of the mutant flagella have bulges filled with electron-dense material (arrowheads in E). The flagellum shown in F also has defects in axonemal doublets and the central pair microtubules; however, such defects are unusual in the mutant, as most axonemal cross sections appear normal. (H) Histogram depicting the distribution of the distances between the transition zone cylinder and the flagellar membrane in wild-type and cep290 mutant cells; the difference in the distributions is significant at P = 0.0025 for a Student’s t test and P = 0.0009 for a Welch’s t test. (I and J) Cells were detergent-extracted, then fixed and processed for EM. The detergent-resistant membrane that remains attached to the wild-type transition zone (arrows in I) is no longer attached to the mutant transition zone. Bar, 100 nm.
Figure 4.
Immunogold localization of CEP290. (A) Wild-type detergent-extracted cytoskeletons were incubated with CEP290 antibody and gold-conjugated secondary antibody, then fixed, embedded, and sectioned. The locations of 34 gold particles from ∼30 sections are indicated by black dots superimposed on the EM. For simplicity, the black dots are depicted on only one side of the transition zone; no bias for either side of the transition zone was observed. A selection of original micrographs is shown in
Fig. S3
. (B and C) cep290::CEP290-HA cytoskeletons were double-labeled with antibodies to CEP290 (12-nm gold) and HA (6-nm gold). As in A, the locations of the particles from several longitudinal sections (B) and cross sections (C) are represented by black dots superimposed on a single EM. A selection of original micrographs is shown in
Fig. S4
. Bars, 100 nm. (D) Schematic of HA-tagged CEP290 depicting the locations of the HA and C-terminal epitopes.
Figure 5.
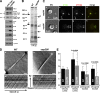
cep290 mutant flagella have an abnormal protein composition. (A) Western blots of isolated flagella show an accumulation of IFT complex B proteins (IFT20, IFT46, and IFT81) and BBS4, and a reduction in the IFT complex A protein IFT139 and polycystin-2 (PKD2). The arrowhead marks an anti–FMG-1-immunoreactive band that is absent in the mutant flagella. (B) Silver-stained gel of isolated wild-type (WT) and mutant (cep290) flagella demonstrating that some proteins are abnormally present (arrows) and others are missing or reduced (arrowheads) in the mutant flagella. Proteins identified by mass spectrometry include (1) flagellar adenylate kinase and FAP5, (2) FAP12, (3) EF-3, and (4) EF-1α1. The positions of standard proteins and their molecular masses in kD are indicated. (C) Immunofluorescence indicates that bulges (arrowheads) in the cep290 mutant flagella contain IFT proteins. (D) DIC imaging and kymographs of IFT in living cells. (D, a and b) Still images of the videos (
Videos 5 and 6
) used to create the kymographs (a′ and b′). Bars, 1 µm. (E) Velocities and frequencies of IFT in wild-type and cep290 mutant flagella. Data are represented as mean ± SD (error bars); P-values were determined using a Student’s t test.
Figure 6.
CEP290 is dynamic and can incorporate into preassembled mutant or wild-type transition zones. (A) Wild-type gametic cells were mated with cep290 mutant gametic cells, and the fused, quadriflagellated cells were detergent-extracted, fixed, and processed for immunofluorescence at the indicated time points after the two cell types were mixed for mating. The quadriflagellated cytoskeletons were double labeled with antibodies to CEP290 and acetylated tubulin. Arrowheads mark the dimmer pair of transition zones. (B) Wild-type gametes were mated to cep290::CEP290-HA gametes and processed as in A except that CEP290-HA was visualized using anti-HA. Bars, 1 µm.
Comment in
- NPHP proteins: gatekeepers of the ciliary compartment.
Omran H. Omran H. J Cell Biol. 2010 Sep 6;190(5):715-7. doi: 10.1083/jcb.201008080. J Cell Biol. 2010. PMID: 20819931 Free PMC article.
Similar articles
- NPHP4 controls ciliary trafficking of membrane proteins and large soluble proteins at the transition zone.
Awata J, Takada S, Standley C, Lechtreck KF, Bellvé KD, Pazour GJ, Fogarty KE, Witman GB. Awata J, et al. J Cell Sci. 2014 Nov 1;127(Pt 21):4714-27. doi: 10.1242/jcs.155275. Epub 2014 Aug 22. J Cell Sci. 2014. PMID: 25150219 Free PMC article. - Centrin plays an essential role in microtubule severing during flagellar excision in Chlamydomonas reinhardtii.
Sanders MA, Salisbury JL. Sanders MA, et al. J Cell Biol. 1994 Mar;124(5):795-805. doi: 10.1083/jcb.124.5.795. J Cell Biol. 1994. PMID: 8120100 Free PMC article. - Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility.
Lechtreck KF, Witman GB. Lechtreck KF, et al. J Cell Biol. 2007 Feb 12;176(4):473-82. doi: 10.1083/jcb.200611115. J Cell Biol. 2007. PMID: 17296796 Free PMC article. - Directed movements of ciliary and flagellar membrane components: a review.
Bloodgood RA. Bloodgood RA. Biol Cell. 1992;76(3):291-301. doi: 10.1016/0248-4900(92)90431-y. Biol Cell. 1992. PMID: 1305476 Review. - Cellular deflagellation.
Quarmby LM. Quarmby LM. Int Rev Cytol. 2004;233:47-91. doi: 10.1016/S0074-7696(04)33002-0. Int Rev Cytol. 2004. PMID: 15037362 Review.
Cited by
- Knockdown of Bardet-Biedl syndrome gene BBS9/PTHB1 leads to cilia defects.
Veleri S, Bishop K, Dalle Nogare DE, English MA, Foskett TJ, Chitnis A, Sood R, Liu P, Swaroop A. Veleri S, et al. PLoS One. 2012;7(3):e34389. doi: 10.1371/journal.pone.0034389. Epub 2012 Mar 29. PLoS One. 2012. PMID: 22479622 Free PMC article. - CEP290 alleles in mice disrupt tissue-specific cilia biogenesis and recapitulate features of syndromic ciliopathies.
Rachel RA, Yamamoto EA, Dewanjee MK, May-Simera HL, Sergeev YV, Hackett AN, Pohida K, Munasinghe J, Gotoh N, Wickstead B, Fariss RN, Dong L, Li T, Swaroop A. Rachel RA, et al. Hum Mol Genet. 2015 Jul 1;24(13):3775-91. doi: 10.1093/hmg/ddv123. Epub 2015 Apr 9. Hum Mol Genet. 2015. PMID: 25859007 Free PMC article. - TMEM231, mutated in orofaciodigital and Meckel syndromes, organizes the ciliary transition zone.
Roberson EC, Dowdle WE, Ozanturk A, Garcia-Gonzalo FR, Li C, Halbritter J, Elkhartoufi N, Porath JD, Cope H, Ashley-Koch A, Gregory S, Thomas S, Sayer JA, Saunier S, Otto EA, Katsanis N, Davis EE, Attié-Bitach T, Hildebrandt F, Leroux MR, Reiter JF. Roberson EC, et al. J Cell Biol. 2015 Apr 13;209(1):129-42. doi: 10.1083/jcb.201411087. J Cell Biol. 2015. PMID: 25869670 Free PMC article. - Cell- and subunit-specific mechanisms of CNG channel ciliary trafficking and localization in C. elegans.
Wojtyniak M, Brear AG, O'Halloran DM, Sengupta P. Wojtyniak M, et al. J Cell Sci. 2013 Oct 1;126(Pt 19):4381-95. doi: 10.1242/jcs.127274. Epub 2013 Jul 25. J Cell Sci. 2013. PMID: 23886944 Free PMC article. - Regulation of centriolar satellite integrity and its physiology.
Hori A, Toda T. Hori A, et al. Cell Mol Life Sci. 2017 Jan;74(2):213-229. doi: 10.1007/s00018-016-2315-x. Epub 2016 Aug 2. Cell Mol Life Sci. 2017. PMID: 27484406 Free PMC article. Review.
References
- Besharse J.C., Horst C.J. 1990. The photoreceptor connecting cilium: a model for the transition zone. Ciliary and Flagellar Membranes. Bloodgood R.A., editor Plenum Press, New York: 389–417
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM030626/GM/NIGMS NIH HHS/United States
- F32 GM087848/GM/NIGMS NIH HHS/United States
- R37 GM030626/GM/NIGMS NIH HHS/United States
- GM030626/GM/NIGMS NIH HHS/United States
- R37 GM014642/GM/NIGMS NIH HHS/United States
- GM014642/GM/NIGMS NIH HHS/United States
- P30 DK032520/DK/NIDDK NIH HHS/United States
- R01 GM014642/GM/NIGMS NIH HHS/United States
- GM087848/GM/NIGMS NIH HHS/United States
- DK32520/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources