Induced loss of ADAR2 engenders slow death of motor neurons from Q/R site-unedited GluR2 - PubMed (original) (raw)
Induced loss of ADAR2 engenders slow death of motor neurons from Q/R site-unedited GluR2
Takuto Hideyama et al. J Neurosci. 2010.
Abstract
GluR2 is a subunit of the AMPA receptor, and the adenosine for the Q/R site of its pre-mRNA is converted to inosine (A-to-I conversion) by the enzyme called adenosine deaminase acting on RNA 2 (ADAR2). Failure of A-to-I conversion at this site affects multiple AMPA receptor properties, including the Ca(2+) permeability of the receptor-coupled ion channel, thereby inducing fatal epilepsy in mice (Brusa et al., 1995; Feldmeyer et al., 1999). In addition, inefficient GluR2 Q/R site editing is a disease-specific molecular dysfunction found in the motor neurons of sporadic amyotrophic lateral sclerosis (ALS) patients (Kawahara et al., 2004). Here, we generated genetically modified mice (designated as AR2) in which the ADAR2 gene was conditionally targeted in motor neurons using the Cre/loxP system. These AR2 mice showed a decline in motor function commensurate with the slow death of ADAR2-deficient motor neurons in the spinal cord and cranial motor nerve nuclei. Notably, neurons in nuclei of oculomotor nerves, which often escape degeneration in ALS, were not decreased in number despite a significant decrease in GluR2 Q/R site editing. All cellular and phenotypic changes in AR2 mice were prevented when the mice carried endogenous GluR2 alleles engineered to express edited GluR2 without ADAR2 activity (Higuchi et al., 2000). Thus, loss of ADAR2 activity causes AMPA receptor-mediated death of motor neurons.
Figures
Figure 1.
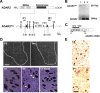
Generation of a conditional ADAR2 knock-out mouse. A, A LoxP site (filled triangle) was inserted into intron 6 and another LoxP site in intron 9 with a selection cassette containing the gene for neomycin resistance (Neo) flanked by FRT sites. Exons are depicted as black bars with numbers. RBDs, RNA binding domains; F1/R1, primer pair (supplemental Table S1, available at
as supplemental material) for B; S, SfiI; BI, BglI; BII, BglII; E, ERI. B, Genomic PCR using template DNA obtained from the tails of ADAR2flox/flox mice (lane 1), ADAR2 flox/+ mice (lane 2), and ADAR2+/+ mice (lane 3). C, Exons excised by recombination are shown as shaded areas in the mRNA, and a black bar indicates the in situ hybridization probe (supplemental Table S1, available at
as supplemental material) for D. F2/R2, Primer pair (supplemental Table S1, available at
as supplemental material) used in Figure 2_B_. D, In situ hybridization using a probe that encompasses the region excised by Cre-mediated recombination. There is a large number of punctate signals in the gray matter (outlined with dotted lines) of control mice (Ctl), whereas nuclei of some large neurons in the anterior horn were devoid of signal in the ADAR2flox/flox/VAChT–Cre.Fast (AR2) mice at 6 months of age (6m; arrowheads in magnified view). The sense probe did not yield a visible signal in the control mice at the same age (Ctl sense). Scale bars: top panels, 200 μm; bottom panels, 25 μm. E, All SMI-32-positive large neurons in the anterior horn (AHCs, brown color in the cytoplasm) of the cervical cord (C5) were ADAR2 positive (dark gray color in the nuclei) in the control mice (Ctl), whereas some of them were devoid of ADAR2 immunoreactivity in AR2 mice at 2 months of age (2m, arrowheads and inset). Sections were counterstained with hematoxylin. Scale bar: 50 μm; inset, 25 μm.
Figure 2.
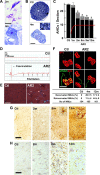
Cre-dependent targeting of ADAR2 and GluR2 Q/R site-editing in motor neurons. A, Frequency histogram of editing efficiency at the GluR2 Q/R site in specimens (lysates containing 3 motor neurons) obtained from AR2 mice at 2 months of age (2m; n = 4). Neurons were dissected with a laser microdissector (inset). B, Specimens (n = 116) were collected into four groups depending on the predicted number of ADAR2-deficient neurons in each specimen; the groups of specimens containing 3, 2, 1, and 0 unedited GluR2-expressing neurons were designated as groups 0:3, 1:2, 2:1, and 3:0, respectively. The ADAR2flox gene and transcripts of the Cre gene and the ADAR2flox alleles before and after recombination were analyzed for each group by PCR. AHCs expressing unedited GluR2 mRNA (group 0:3) harbored the truncated ADAR2flox gene and Cre transcripts, whereas AHCs expressing edited GluR2 mRNA (group 3:0) carried the full-length ADAR2flox gene and did not express Cre. Ctl1, ADAR2flox/flox mice; Ctl2, VAChT–Cre.Fast mice; AH, anterior horn of the spinal cord.
Figure 3.
Behavioral changes in AR2 mice. A, Rotarod performance presented as latency to fall (at 10 rpm, 180 s at the maximum) began to decline at 5 weeks of age in AR2 mice and rapidly fell to low levels during the initial 5–6 months, remaining stable until 18 months of age. Control mice exhibited full performance (180 s) until ∼12 months of age, followed by slightly lower performance (>164.5 ± 6.4 s) until 24 months. B, Grip strength measured declined with kinetics similar to those of rotarod performance. In A and B, the scores obtained for the AR2 mice (mean ± SEM; n = 28) are indicated as percentage performance of control mice (n = 15). C, AR2 mice exhibited slightly lower body weight than controls (p > 0.05). D, AR2 mice (n = 33) had long lifespans, but the rate of death increased after month 18. The median ± SEM survival was 81.5 ± 16.4 weeks for AR2 mice compared with 105.1 ± 13.5 weeks for control mice (p = 0.0262, log-rank analysis).
Figure 4.
Loss of ADAR2-deficient motor neurons. A, Degenerating AHCs in AR2 mice at 2 months (2m; Nissl staining) and 4 months (4m; toluidine blue staining, 1 μm section) of age. Scale bar: 2m, 25 μm; 4m, 12.5 μm. B, Ventral root (L5) of control (Ctl) and AR2 mice at 4 months of age (4m). Inset, Magnified view of degenerating axons. Scale bar: 100 μm; inset, 20 μm. C, Numbers of AHCs showing ADAR2 immunoreactivity (black columns) and lacking this immunoreactivity (gray columns) (mean ± SEM) in AR2 mice at different ages (1m, 2m, 6m, 9m, 12m). In AR2 mice, Cre expression is developmentally regulated (orange line), and ∼50% of motor neurons express Cre by 5 weeks of age, with recombination of the ADAR2 gene in ∼10% of AHCs at 1 month of age and 40–45% of AHCs after 2 months of age (orange line). The number of ADAR2-lacking AHCs significantly decreased in AR2 mice after 2 months of age as a result of Cre-dependent knock-out of ADAR2 (*p < 0.01, repeated-measures ANOVA). The number of AHCs in the control mice did not change at different ages, and all the AHCs in controls showed ADAR2 immunoreactivity. D, Electrophysiological examination in AR2 mice. Electromyography from an AR2 mouse at 12 months of age showing fibrillations and fasciculations, common findings in ALS indicative of muscle fiber denervation and motor unit degeneration and regeneration. These findings were observed in two other AR2 mice examined but never in control mice (Ctl; n = 2). E, Calf muscles from a wild-type mouse (left) and an AR2 mouse (middle and right) at 12 months of age. Characteristics of denervated muscles, including muscle fiber atrophy (white arrow), centrally placed nuclei, and pyknotic nuclear clumps (white arrowhead) are observed in the AR2 mouse. Hematoxylin and eosin. Scale bar, 60 μm. F, NMJs and distal axons. Quadriceps muscles from a wild-type mouse (Ctl; left) and an AR2 mouse (AR2; middle and right) at 12 months of age are stained with tetramethylrhodamine–bungarotoxin (BTX) (red) and immunostained concomitantly with anti-synaptophysin and neurofilament (SYN/NF) antibodies (green). Endplates (red) were counted as “innervated“ if they were merged with axon terminals (merge; yellow). Each endplate is innervated by a thick axon terminal in the Ctl mouse. In AR2 mice, in addition to the normally innervated NMJs, some NMJs were innervated by axons that simultaneously innervate more than one NMJ (reinnervated NMJs; middle), and other NMJs were devoid of axon terminals (denervated NMJs; right). More than 50 NMJs were counted in each animal in the control group and groups of AR2 mice at 4 and 12 months of age (n = 3 in each group). Proportions of denervated NMJs and reinnervated NMJs among total NMJs in each group are indicated as mean ± SD (percentage). Scale bar, 25 μm. G, H, Immunohistochemistry in the anterior horn (C5). There was a time-dependent increase in GFAP immunoreactivity (G) and an increase in MAC2 immunoreactivity maximal at 6 months of age (H) in the spinal anterior horn of AR2 mice. m, Months of age; inset, activated astroglia. Scale bars: G, 100 μm; insets and H, 50 μm.
Figure 5.
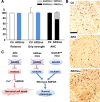
Crucial role of GluR2 Q/R site editing in death of ADAR2-deficient motor neurons. A, AR2/GluR-BR/R mice (AR2res) displayed full rotarod score and normal grip strength at 6 months of age compared with control mice (Ctl). The number of total AHCs, of which a considerable proportion was deficient in ADAR2, did not decrease in AR2res mice. B, At 6 months of age, although only a few AHCs lacking ADAR2 immunoreactivity (arrowheads) were observed in AR2 mice, a considerable number of AHCs lacking ADAR2 immunoreactivity was present in AR2res mice. The density of AHCs in AR2res mice was similar to that in the control mice in which all the AHCs were immunoreactive to ADAR2 in their nuclei. Sections were counterstained with hematoxylin. Scale bar, 100 μm. C, Scheme illustrating that lack of ADAR2 induces slow death of motor neurons in AR2 mice but not in AR2res mice that express Q/R site-edited GluR2 in the absence of ADAR2 activity. The exonic Q codon at the Q/R site of GluR2 was substituted by an R codon in the endogenous GluR2 alleles of GluR-BR/R mice.
Comment in
- Commentary (two hits with one shot: a possibility of simultaneous targeting motor neuron loss and depression in ALS by upregulating ADAR2).
Siklos L. Siklos L. CNS Neurol Disord Drug Targets. 2011 Dec;10(8):863. doi: 10.2174/187152711799219389. CNS Neurol Disord Drug Targets. 2011. PMID: 22229319 No abstract available.
Similar articles
- The molecular link between inefficient GluA2 Q/R site-RNA editing and TDP-43 pathology in motor neurons of sporadic amyotrophic lateral sclerosis patients.
Yamashita T, Kwak S. Yamashita T, et al. Brain Res. 2014 Oct 10;1584:28-38. doi: 10.1016/j.brainres.2013.12.011. Epub 2013 Dec 16. Brain Res. 2014. PMID: 24355598 Review. - Profound downregulation of the RNA editing enzyme ADAR2 in ALS spinal motor neurons.
Hideyama T, Yamashita T, Aizawa H, Tsuji S, Kakita A, Takahashi H, Kwak S. Hideyama T, et al. Neurobiol Dis. 2012 Mar;45(3):1121-8. doi: 10.1016/j.nbd.2011.12.033. Epub 2011 Dec 28. Neurobiol Dis. 2012. PMID: 22226999 - Negative features of sporadic amyotrophic lateral sclerosis: Motor neurons of Onuf's nucleus survive in ADAR2-conditional knockout mice.
Hideyama T, Teramoto S, Kato H, Terashi H, Kwak S, Aizawa H. Hideyama T, et al. J Neurol Sci. 2024 Aug 15;463:123142. doi: 10.1016/j.jns.2024.123142. Epub 2024 Jul 15. J Neurol Sci. 2024. PMID: 39053342 - Deficient RNA editing of GluR2 and neuronal death in amyotropic lateral sclerosis.
Kwak S, Kawahara Y. Kwak S, et al. J Mol Med (Berl). 2005 Feb;83(2):110-20. doi: 10.1007/s00109-004-0599-z. Epub 2004 Dec 29. J Mol Med (Berl). 2005. PMID: 15624111 Review. - Novel etiological and therapeutic strategies for neurodiseases: RNA editing enzyme abnormality in sporadic amyotrophic lateral sclerosis.
Hideyama T, Yamashita T, Nishimoto Y, Suzuki T, Kwak S. Hideyama T, et al. J Pharmacol Sci. 2010;113(1):9-13. doi: 10.1254/jphs.09r21fm. Epub 2010 Apr 16. J Pharmacol Sci. 2010. PMID: 20424386 Review.
Cited by
- Activity-dependent A-to-I RNA editing in rat cortical neurons.
Sanjana NE, Levanon EY, Hueske EA, Ambrose JM, Li JB. Sanjana NE, et al. Genetics. 2012 Sep;192(1):281-7. doi: 10.1534/genetics.112.141200. Epub 2012 Jun 19. Genetics. 2012. PMID: 22714409 Free PMC article. - Ocular Hypertension Drives Remodeling of AMPA Receptors in Select Populations of Retinal Ganglion Cells.
Sladek AL, Nawy S. Sladek AL, et al. Front Synaptic Neurosci. 2020 Jul 24;12:30. doi: 10.3389/fnsyn.2020.00030. eCollection 2020. Front Synaptic Neurosci. 2020. PMID: 32792936 Free PMC article. - RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention.
Donnelly CJ, Zhang PW, Pham JT, Haeusler AR, Mistry NA, Vidensky S, Daley EL, Poth EM, Hoover B, Fines DM, Maragakis N, Tienari PJ, Petrucelli L, Traynor BJ, Wang J, Rigo F, Bennett CF, Blackshaw S, Sattler R, Rothstein JD. Donnelly CJ, et al. Neuron. 2013 Oct 16;80(2):415-28. doi: 10.1016/j.neuron.2013.10.015. Neuron. 2013. PMID: 24139042 Free PMC article. - Ion channel dysfunction and altered motoneuron excitability in ALS.
LoRusso E, Hickman JJ, Guo X. LoRusso E, et al. Neurol Disord Epilepsy J. 2019;3(2):124. Epub 2019 Jul 30. Neurol Disord Epilepsy J. 2019. PMID: 32313901 Free PMC article. - NMJ-related diseases beyond the congenital myasthenic syndromes.
Navarro-Martínez A, Vicente-García C, Carvajal JJ. Navarro-Martínez A, et al. Front Cell Dev Biol. 2023 Aug 4;11:1216726. doi: 10.3389/fcell.2023.1216726. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37601107 Free PMC article. Review.
References
- Akbarian S, Smith MA, Jones EG. Editing for an AMPA receptor subunit RNA in prefrontal cortex and striatum in Alzheimer's disease, Huntington's disease and schizophrenia. Brain Res. 1995;699:297–304. - PubMed
- Beleza-Meireles A, Al-Chalabi A. Genetic studies of amyotrophic lateral sclerosis: controversies and perspectives. Amyotroph Lateral Scler. 2009;10:1–14. - PubMed
- Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, Seeburg PH, Sprengel R. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–1680. - PubMed
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous