Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling - PubMed (original) (raw)
Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling
Arthit Chairoungdua et al. J Cell Biol. 2010.
Abstract
CD82 and CD9 are tetraspanin membrane proteins that can function as suppressors of tumor metastasis. Expression of CD9 and CD82 in transfected cells strongly suppresses β-catenin-mediated Wnt signaling activity and induces a significant decrease in β-catenin protein levels. Inhibition of Wnt/β-catenin signaling is independent of glycogen synthase kinase-3β and of the proteasome- and lysosome-mediated protein degradation pathways. CD82 and CD9 expression induces β-catenin export via exosomes, which is blocked by a sphingomyelinase inhibitor, GW4869. CD82 fails to induce exosome release of β-catenin in cells that express low levels of E-cadherin. Exosome release from dendritic cells generated from CD9 knockout mice is reduced compared with that from wild-type dendritic cells. These results suggest that CD82 and CD9 down-regulate the Wnt signaling pathway through the exosomal discharge of β-catenin. Thus, exosomal packaging and release of cytosolic proteins can modulate the activity of cellular signaling pathways.
Figures
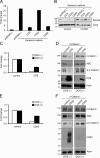
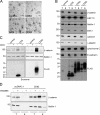
Figure 1.
Effects of tetraspanins on TCF/LEF reporter activity and levels of β-catenin. (A) CD9 and CD82 expression inhibits β-catenin signaling. HEK 293T cells were transiently cotransfected with TOPflash, Xpress-tagged β-catenin, the indicated tetraspanins, and the Renilla luciferase vector. Activity of the β-catenin signaling pathway was quantified by measuring relative firefly luciferase activity units (RLUs) normalized to Renilla luciferase. Data are presented as the fold change as compared with the control condition (cells transfected with pcDNA3.1 empty vector alone). (B) CD9 and CD82 expression reduces cytosolic and nuclear pools of β-catenin. HEK 293T cells were harvested 24 h after transient transfection with the indicated plasmids. Cell lysates were analyzed by Western blotting using anti-Xpress (β-catenin) and anti–β-actin antibodies. (C–F) Inducible expression of tetraspanins inhibits the activity of β-catenin–mediated signaling. HEK 293T cells were transiently cotransfected with a cDNA encoding CD9 or CD82 under the control of a Tet-Off promoter, TOPflash, β-catenin, and Renilla luciferase vector. After culture in the presence (DOX (+)) and absence (DOX (−)) of 100 ng/ml of doxycycline for 48 h, cells were harvested for luciferase-based TOPflash assay of Wnt signaling (C and E) and immunoblotted with the indicated antibodies (D and F). Luciferase data are presented as the fold change as compared with the control condition (untransfected cells).
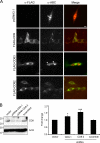
Figure 2.
Localization of β-catenin in CHO cells. (A) CHO cells were transfected with the indicated plasmids and immunostained for tetraspanins and β-catenin using an anti-FLAG and anti-dephosphorylated β-catenin (anti-ABC), respectively. In control and CD63-transfected cells, β-catenin immunostaining was predominantly localized in the nucleus (arrowhead). Upon coexpression with CD9 and CD82, β-catenin staining was decreased in the nucleus and was associated instead with a punctuate plasma membrane pattern. Bar, 10 µm. (B) Knockdown of CD9 stimulates Wnt/β-catenin signaling. HeLa cells were cotransfected with shRNA targeted for CD9 sequences or with a scrambled control sequence as described in Materials and methods, as well as with plasmids encoding β-catenin, TOPflash, and Renilla luciferase. After 48 h cells were harvested and analyzed by Western blotting using anti-dephosphorylated β-catenin (anti-ABC) and anti-CD9. β-Actin was used as a loading control (left). Corresponding lysates were used for luciferase assay (right). Data are shown as the fold change compared with control (cells transfected with empty vector). Data are presented as mean ± SEM. *, P < 0.05; ***, P < 0.005 compared with control (t test).
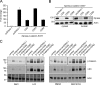
Figure 3.
CD9 and CD82 inhibition of Wnt/β-catenin signaling is GSK-3β independent. (A) CD9 and CD82 but not CD63 inhibit TOPflash activity stimulated by mutant S33Y–β-catenin. 24 h after transfection with the indicated plasmids, cells were harvested for TOPflash luciferase assay. Data are presented as the fold change as compared with the control condition (cells transfected with pcDNA3.1 empty vector alone). (B) CD9 and CD82 expression reduced cytosolic and nuclear pools of the mutant S33Y–β-catenin protein. Cell fractions prepared from HEK 293T cells transfected with the indicated plasmids were immunoblotted using anti-Xpress (β-catenin) and anti–β-actin antibodies. (C) Effects of GSK-3β inhibitors on β-catenin levels. 12 h after transfection with the indicated plasmids, HEK 293T cells were treated with 50 mM NaCl, 50 mM LiCl, 20 µM SB216763, or DMSO (control) for 12 h. Lysates were blotted with anti–β-catenin and anti-dephosphorylated β-catenin (anti-ABC). β-Actin was used as a loading control.
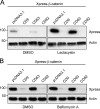
Figure 4.
CD9- and CD82-mediated β-catenin destabilization is independent from the proteasome- and lysosome-associated protein degradation pathways. HEK 293T cells cotransfected with the indicated plasmids were treated with 10 µM _clasto_-lactacystin (A), 250 nM bafilomycin A (B), or DMSO vehicle alone for 16 h before being harvested for Western blotting using anti-Xpress antibody (β-catenin). β-Actin was used as a loading control. CD9 and CD82 were still able to reduce β-catenin protein levels in the presence of both inhibitors.
Figure 5.
β-catenin is secreted into exosomes. (A) Electron microscopy of extracellular exosomes secreted by HEK 293T cells. Exosomes were isolated by sequential centrifugation steps as described in Materials and methods from HEK 293T cells transfected with the indicated plasmids. Control cells were transfected with empty vector. Bar, 200 nm. (B) β-Catenin, E-cadherin, and tetraspanins are secreted into exosomes. Total lysate (T) and exosome (E) fractions purified from HEK 293T cells transfected with the indicated plasmids were analyzed by Western blotting using the indicated antibodies. EEA1, calnexin, and γ-adaptin are membrane markers for early endosomes, ER, and TGN, respectively. Cytochrome c was used as a marker for apoptotic cell fragments. (C) Exosome release of β-catenin is dependent on CD9 and CD82. HEK 293T cells were transiently transfected with a cDNA encoding CD9 and CD82 under control of the Tet-Off promoter or empty vector as control. After being cultured in the presence (DOX (+)) and absence (DOX (−)) of 100 ng/ml of doxycycline for 48 h, total lysate (T) and exosome (E) fractions were analyzed by Western blotting using the indicated antibodies. The exosomal protein, flotillin, was used as a loading control. (D) Exosome release of β-catenin is reduced after treatment with a sphingomyelinase (nSMase) inhibitor. HEK 293T cells were transfected with CD82 or with empty vector as control and then treated with 5 µM GW4869 or DMSO for 16 h. Total lysate (T) and exosome (E) fractions were blotted with anti–β-catenin and anti–flotillin-1 antibodies.
Figure 6.
E-cadherin–dependent exosome release of β-catenin. (A) CD82 interacts with the E-cadherin–β-catenin complex. HEK 293T cells stably expressing CD82 or empty vector were transfected with E-cadherin-GFP and β-catenin. 24 h after transfection, cells were lysed with the indicated lysis buffer and immunoprecipitated with anti-FLAG M2 affinity gel. The immunoprecipitated samples were washed with the indicated concentration of NaCl, followed by Western blotting with anti-GFP and anti–β-catenin antibodies (left). Total cell lysates were subjected to Western blotting with the indicated antibodies (right). (B) CD82 interacts with the E-cadherin–β-catenin complex in exosomes. Exosomes were purified from HEK 293T cells stably expressing CD82 or empty vector. Exosome fractions were lysed with 0.3% CHAPS lysis buffer and immunoprecipitated with anti-FLAG M2 affinity gel. The immunoprecipitated samples were washed with lysis buffer, followed by Western blotting with the indicated antibodies. (C) E-cadherin is required for CD82-induced exosome release of β-catenin. A431D cells were cotransfected with the indicated plasmids. After 48 h, total lysate and exosome fractions were purified and analyzed by Western blotting using the indicated antibodies.
Figure 7.
Effects of CD9 on Wnt/β-catenin signaling and exosome release in vivo. (A) Reduction of BMDC-derived exosomes from CD9 knockout mice. Exosomes were purified from BMDCs derived from CD9 wild-type and knockout mice as described in Materials and methods and analyzed by Western blotting using the indicated antibodies. BMDC lysates were immunoblotted with anti-CD9 as shown in the bottom panel. (B) CD82 expression inhibits β-catenin signaling and facilitates the exosome release of β-catenin in PC3 cells. PC3 cells were transiently cotransfected with the indicated plasmids and with the TOPflash and the Renilla luciferase vectors. Activity of the β-catenin signaling pathway was quantified by measuring relative firefly luciferase activity units (RLUs) normalized to Renilla luciferase and expressed as fold change in the luciferase signal (top panel). In the bottom panel, Western blots of total lysates and exosome fractions were purified from PC3 cells transiently transfected with indicated plasmids. CD82 expression increased the exosome release of β-catenin by a factor of 3. (C) A novel role for tetraspanins in the regulation of Wnt/β-catenin signaling. At steady state, tetraspanins are organized in a signaling complex with E-cadherin at the plasma membrane. This signaling complex, including tetraspanins, E-cadherin, and β-catenin, is internalized and delivered to early endosomes. Exosome biogenesis begins with outward vesicle budding at the limiting membrane of endosomes, generating intraluminal vesicles (ILVs). These exosome-containing endosomes eventually mature into late endosomes, also known as mutivesicular bodies (MVBs). These MVBs then fuse with plasma membrane and release their intraluminal vesicles, referred to as exosomes, which contain β-catenin. The exosome targeting of β-catenin causes a reduction in the intracellular pool of β-catenin and therefore reduces Wnt/β-catenin signaling.
Comment in
- Tumour suppression: Ejector seat.
McCarthy N. McCarthy N. Nat Rev Cancer. 2010 Nov;10(11):739. doi: 10.1038/nrc2951. Nat Rev Cancer. 2010. PMID: 21080585 No abstract available.
Similar articles
- CD82 inhibits canonical Wnt signalling by controlling the cellular distribution of β-catenin in carcinoma cells.
Chigita S, Sugiura T, Abe M, Kobayashi Y, Shimoda M, Onoda M, Shirasuna K. Chigita S, et al. Int J Oncol. 2012 Dec;41(6):2021-8. doi: 10.3892/ijo.2012.1671. Epub 2012 Oct 17. Int J Oncol. 2012. PMID: 23076981 Free PMC article. - Tetraspanin CD82 represses Sp1-mediated Snail expression and the resultant E-cadherin expression interrupts nuclear signaling of β-catenin by increasing its membrane localization.
Lee MS, Byun HJ, Lee J, Jeoung DI, Kim YM, Lee H. Lee MS, et al. Cell Signal. 2018 Dec;52:83-94. doi: 10.1016/j.cellsig.2018.09.001. Epub 2018 Sep 4. Cell Signal. 2018. PMID: 30189244 - Nuclear GSK-3beta inhibits the canonical Wnt signalling pathway in a beta-catenin phosphorylation-independent manner.
Caspi M, Zilberberg A, Eldar-Finkelman H, Rosin-Arbesfeld R. Caspi M, et al. Oncogene. 2008 Jun 5;27(25):3546-55. doi: 10.1038/sj.onc.1211026. Epub 2008 Jan 28. Oncogene. 2008. PMID: 18223684 - The calpain system is involved in the constitutive regulation of beta-catenin signaling functions.
Benetti R, Copetti T, Dell'Orso S, Melloni E, Brancolini C, Monte M, Schneider C. Benetti R, et al. J Biol Chem. 2005 Jun 10;280(23):22070-80. doi: 10.1074/jbc.M501810200. Epub 2005 Apr 7. J Biol Chem. 2005. PMID: 15817486 - Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity.
Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, Hung MC, Bresalier RS. Song S, et al. Cancer Res. 2009 Feb 15;69(4):1343-9. doi: 10.1158/0008-5472.CAN-08-4153. Epub 2009 Feb 3. Cancer Res. 2009. PMID: 19190323 Free PMC article.
Cited by
- Influence of inflammation on the expression of microRNA-140 in extracellular vesicles from 2D and 3D culture models of synovial-membrane-derived stem cells.
Pfeifer JPH, Stievani FC, Fernandes CJDC, Rosa GDS, Apolonio EVP, Rossi MC, Zambuzzi WF, Alves ALG. Pfeifer JPH, et al. Front Bioeng Biotechnol. 2024 Aug 7;12:1416694. doi: 10.3389/fbioe.2024.1416694. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39170063 Free PMC article. - Exosomes: Methods for Isolation and Characterization in Biological Samples.
Singh S, Dansby C, Agarwal D, Bhat PD, Dubey PK, Krishnamurthy P. Singh S, et al. Methods Mol Biol. 2024;2835:181-213. doi: 10.1007/978-1-0716-3995-5_17. Methods Mol Biol. 2024. PMID: 39105917 Review. - Acidity and hypoxia of tumor microenvironment, a positive interplay in extracellular vesicle release by tumor cells.
Peppicelli S, Calorini L, Bianchini F, Papucci L, Magnelli L, Andreucci E. Peppicelli S, et al. Cell Oncol (Dordr). 2024 Jul 18. doi: 10.1007/s13402-024-00969-z. Online ahead of print. Cell Oncol (Dordr). 2024. PMID: 39023664 Review. - Emerging role of oncogenic ß-catenin in exosome biogenesis as a driver of immune escape in hepatocellular carcinoma.
Dantzer C, Vaché J, Brunel A, Mahouche I, Raymond AA, Dupuy JW, Petrel M, Bioulac-Sage P, Perrais D, Dugot-Senant N, Verdier M, Bessette B, Billottet C, Moreau V. Dantzer C, et al. Elife. 2024 Jul 15;13:RP95191. doi: 10.7554/eLife.95191. Elife. 2024. PMID: 39008536 Free PMC article. - The role of exosomal molecular cargo in exosome biogenesis and disease diagnosis.
Liu M, Wen Z, Zhang T, Zhang L, Liu X, Wang M. Liu M, et al. Front Immunol. 2024 Jun 25;15:1417758. doi: 10.3389/fimmu.2024.1417758. eCollection 2024. Front Immunol. 2024. PMID: 38983854 Free PMC article. Review.
References
- Boucheix C., Duc G.H., Jasmin C., Rubinstein E. 2001. Tetraspanins and malignancy. Expert Rev. Mol. Med. 2001:1–17 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 DK057328/DK/NIDDK NIH HHS/United States
- DK-17433/DK/NIDDK NIH HHS/United States
- DK-57328/DK/NIDDK NIH HHS/United States
- P01 DK017433/DK/NIDDK NIH HHS/United States
- R01 DK072614/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous